周围神经损伤
-
Figure 1|Preparation of the median and sural nerves and of nerve fragments for staining.

Median nerves and sural nerves were obtained from two cadavers at the Korea Public Tissue Bank. Both the body donation agreement and the human body research agreement were obtained from the National Public Tissue Bank. The isolation of the median nerves from both arms and the sural nerves from both legs was performed at the Korea Public Tissue Bank. We obtained two median nerves (20 cm long) and two sural nerves (50 cm long) from a male cadaver (47 years old) and two median nerves (40 cm long) and two sural nerves (30 cm long) from a female cadaver (23 years old). The median and sural nerves were 3.0–3.8 mm and 1.2–1.5 mm in diameter, respectively (Figure 1). The median and sural nerves were cut into 2.0–2.3 cm segments. The 24 segments of the median nerves and 32 segments of the sural nerves were placed separately in a 15-mL tube containing PBS. We divided them into six groups: (A) fresh median nerve, (B) median nerve decellularized by nonionic and anionic detergent (Triton X-100 and sodium deoxycholate), (C) median nerve decellularized by amphoteric detergent and nuclease (CHAPS, deoxyribonuclease I and ribonuclease A), (D) fresh sural nerve, (E) sural nerve decellularized by nonionic and anionic detergent (Triton X-100 and sodium deoxycholate), and (F) sural nerve decellularized by detergent amphoteric and nuclease (CHAPS, deoxyribonuclease I and ribonuclease A).
Figure 2|Hematoxylin-eosin staining results of median and sural nerves decellularized by two different methods.
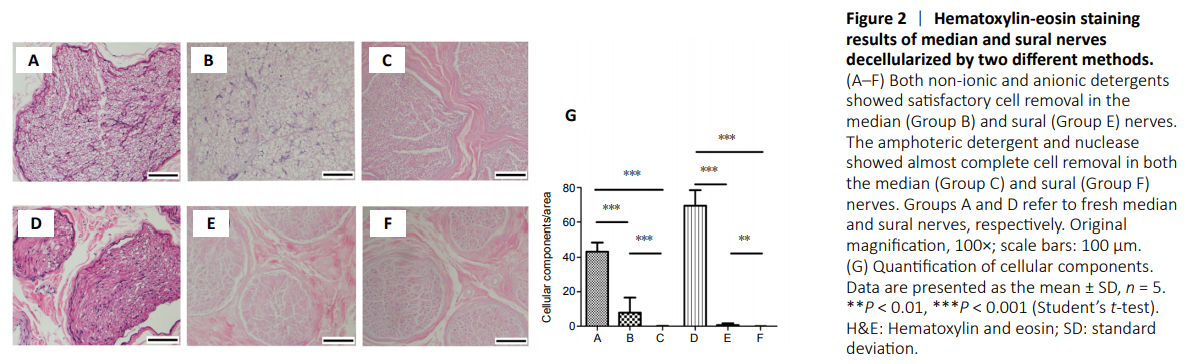
Figure 3|Comparison of DAPI staining of median and sural nerves between two decellularization methods.

Figure 4|DNA content and S-100 immunohistochemistry of median and sural nerves decellularized by two different methods.

The remaining cellular components were identified by H&E staining (Figure 2). Using nonionic and anionic detergent, the cellular components were well removed from the sural nerve (Figure 2E), whereas the cellular components remained in the median nerve (Figure 2B). Groups C and F using amphoteric detergent and nuclease showed almost complete removal of cells. The cellular components visible after DAPI staining are shown in Figure 3. The quantified values of H&E and DAPI staining, with cell removal, showed similar trends in each group. In the DAPI analysis, groups C and F using amphoteric detergent and nucleases and group E using nonionic and anionic detergent showed almost complete removal of cells (Figure 3). Group B showed significantly higher cellular components than the other median nerve groups. The DNA contents of groups C and F were significantly lower than in the other groups treated with nonionic and anionic detergent method (Figure 4A).
In order to compare the degree of removal of Schwann cells, immunohistochemical staining was performed using the S-100 antibody. Results from decellularization with nonionic and anionic detergent showed that a considerable number of Schwann cells remained in the median nerve and partially stained cells in the sural nerve. On the other hand, Schwann cells were removed completely from both the median and sural nerves using the amphoteric detergent and nuclease decellularization method (Figure 4B–G).
Figure 5|Immunostaining against laminin of median nerves and sural nerves decellularized by two different methods.

Figure 6|Comparison of collagen content on median and sural nerves decellularized using two different methods after collagen myelin staining.

The mean scores for basal lamina using laminin immunohistochemical staining are shown in Figure 5. The structural integrity of median and sural nerves decellularized using amphoteric detergent and nuclease was better preserved than that of median and sural nerves decellularized using nonionic and anionic detergent (Figure 5). The collagen content of groups B and E was significantly lower than that of groups A and D (Figure 6). The collagen content in the median and sural nerves decellularized by the amphoteric detergent and nuclease was slightly, but not significantly, lower than that in the fresh median and sural nerves (Figure 6A).
MT staining was performed to visualize the distribution of collagen in the median and sural nerves. Blue collagen, black nuclei, and red cytoplasm were observed in the fresh median and sural nerve. Result of MT staining showed that a larger amount of blue collagen was preserved in the groups C and F, in which amphoteric detergent and nuclease were used, than that in the groups B and E, in which the nonionic and anionic detergents were used (Figure 6B–G). The distribution of the myelin sheath and neurons was compared with Luxol Fast blue-cresyl violet staining. In the fresh median and sural nerve, myelin stained blue green and neurons stained violet were observed. Significantly reduced myelin was observed in the groups C and F (Figure 6H–M).
Figure 7| Contact cytotoxicity assay of sural nerve decellularized with amphoteric detergent and nuclease.
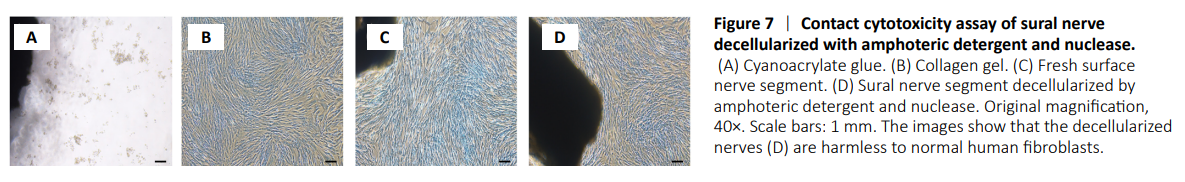
Analysis of contact cytotoxicity revealed that inhibition of cell proliferation or lysis was not observed in either fresh or decellularized sural nerve fragments (Figure 7).