脊髓损伤
-
Figure 1|Measured osmolality of rat serum samples and other saline solutions.
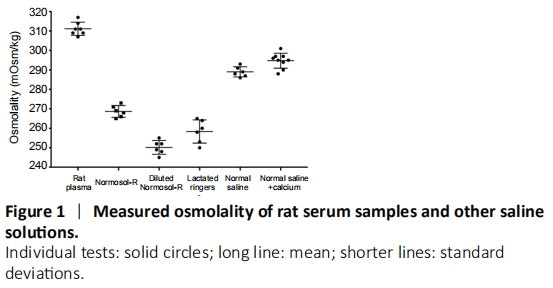
A freezing point osmometer was used to measure osmolality of various saline solutions in this study. Before testing each solution, the device was calibrated using a 290 mOsm/kg reference solution to ensure its accuracy. After calibration, 20 μL of each sample was loaded into the sample plunger. Measurements (Figure 1 and Table 2) were taken for rat serum (n = 7) via intracardiac puncture (Stare and Bourque, 2016). Normosol-R (n = 6) and lactated Ringer’s (n = 6) measurements were taken from two separate bags of solution (3 measures each) at room temperature. Diluted Normosol-R (n = 6) was measured from bags of Normosol-R diluted with ddH2O to ~250 mOsm. Normal saline (n = 6) and normal saline + calcium chloride (n = 9) were made by mixing appropriate salts in ddH2O (Table 2) until fully dissolved and measured at room temperature.
Figure 2|Intraoperative images of sciatic nerves stained with 1% and 0.5% MB.
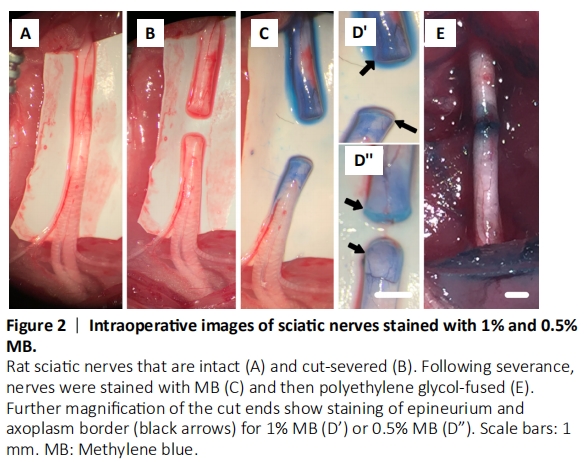
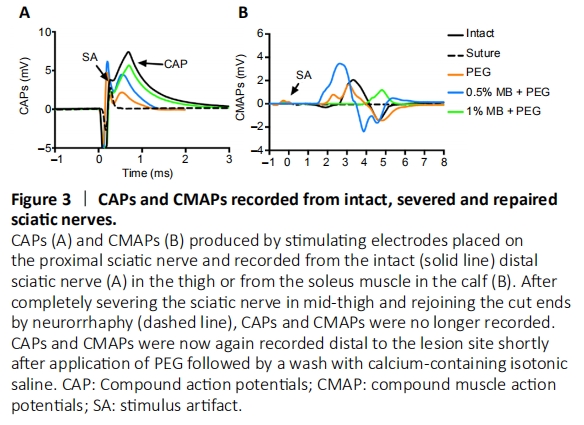
Figure 3|CAPs and CMAPs recorded from intact, severed and repaired sciatic nerves.
Rats were anesthetized using a small mammalian anesthetic device (Handlebar Anesthesia, Austin, TX, USA). Animals were initially induced to anesthesia with 4% inhaled isoflurane (Patterson Veterinary, Greeley, CO, USA), and kept at 2–2.5% for the duration of the surgery. An oxygen flow rate of 0.5–1 L/min was maintained at all times. Prior to surgery, the lateral aspect of the left hindlimb was trimmed of fur and disinfected with alternating swabs of 10% iodine/povidone and 70% ethyl alcohol. Ophthalmic ointment was applied to each eye to prevent drying. A 2–3 cm incision was then made through the skin in the mid-thigh to expose the underlying musculature. A small incision was made parallel to the muscle fibers overlying the sciatic nerve, and a blunt dissection using scissors exposed the intact sciatic nerve (Figure 2A) in extracellular fluid and kept moist with isotonic normal saline supplemented with calcium (Table 2). To confirm that the sciatic nerve and NMJs remained functional, we recorded CAPs by stimulating the nerve proximally with extracellular electrodes placed on the nerve in the proximal thigh and recorded by electrodes (ADInstruments, Sydney, Australia), placed distally on the nerve; CMAPs were recorded from recording electrodes placed in the tibialis anterior muscle. CAPs and CMAPs are bimodal (yes/no) assessments as differences in waveform are mostly a result of electrode placement (Bittner et al, 2015; Ghergherehchi et al., 2016, 2019a, b; Mikesh et al., 2018a, b).
The sciatic nerve bathed in isotonic extracellular fluid or Normal saline + calcium chloride was then completely transected with sharp microscissors in mid-thigh between the two electrodes; the proximal and distal cut ends of the sciatic nerve typically separated by 2–3 mm (Figure 2B). Once the sciatic nerve was severed CAPs and CMAPs were no longer recordable (Figure 3).
As summarized in Table 3, our PEG-fusion protocol to repair a singly transected sciatic nerve consisted of the following sequential steps # (1) – (5):
(1) The proximal and distal severed axonal ends were directly irrigated with a priming solution (Ca2+-free, hypotonic saline: Normosol-R (Hospira, Lake Forest, IL, USA; diluted with ddH2O to ~250 mOsm) for 2–3 minutes to open the axonal ends, expel existing intra-axonal vesicles, and inhibit new vesicle formation that decrease PEG-fusion success.
(2) MB (Acros Organics, Morris Plains, NJ, USA), an anti-oxidant, in distilled water was applied directly to the lesion site for 2–3 minutes to further open axonal ends and inhibit new vesicle formation (Figure 2C; Spaeth et al., 2012; Vargas and Bittner, 2019). We have historically used 1% MB in sterile H2O (Figure 2D’), but 1% MB is no longer readily available for clinical procedures. However, 0.5% MB is now readily available as ProvayBlueTM (American Regent, Shirley, NY, USA) (Figure 2D”). Animals in these groups that also receive PEG (see step (4) below) are referred to as PEG + 1% MB and PEG + 0.5% MB, respectively.
(3) Open, vesicle-free donor and/or host axons and their sheaths were well trimmed at all lesion sites and all axons were closely apposed with microsutures (neurorrhaphy). The microsutures provide mechanical strength at the lesion sites to prevent PEG-fused axons from pulling apart because axonal (or other cell-type) plasmalemmas have minimal tensile strength. Microsutures also help align the two cut ends in their approximate original orientation, but do not re-appose the two cut ends of each (or possibly any) specific axon.
(4) A high concentration (50% w/w solution) of the membrane fusogen PEG (Sigma Alrich, St. Louis, MO, USA; 3.35 kDa PEG in distilled water) was directly applied for 1–2 minutes to non-specifically connect/join (PEG-fuse) the closely-apposed axonal membranes (axolemmas) of open, vesicle-free, distal and proximal axons. The PEG concentration, molecular weight, and time of direct application are critical variables. Experimental animals in this group that receive PEG alone and (no MB, see step (2)) are referred to as PEG animals in this paper.
(5) Several washes of Normal Saline + Calcium chloride that mimicked the host’s extracellular fluids/blood plasma was directly applied to remove all PEG, induce newly-formed vesicles to seal any remaining plasmalemmal holes, and restore axolemmal/axoplasmic continuity of rat sciatic nerves in the upper thigh (Figure 2E). We have historically used Lactated Ringer’s (Hospira, Lake Forest, IL, USA) that calculated as 273 mOsm. However, Lactated Ringer’s measured 258.3 ± 5.9 mOsm when recently assayed with a well-calibrated osmometer, i.e., some ionic particles are not completely dissociated (hydrated). Hence, in this study we used a normal saline with physiological levels of calcium but a different formulation, i.e. 0.9% NaCl + 0.02% CaCl2. This solution calculates as 310 mOsm, but measures 294.7 ± 3.8 mOsm, i.e., isotonic calcium-containing saline. We confirmed that PEG-fusion was initially successful by recording CAPs and CMAPs from PEG, PEG + 0.5% MB, and PEG + 1% MB animals (Figure 3).
Figure 4|SFI scores of control and experimental groups.

The PEG-containing solution in step #4 above was omitted for “NC animals” that never exhibited rapid re-innervation, immediate axonal restoration of through conduction of action potentials (Figure 3) and behavioral recovery within several weeks (Figure 4).
Figure 5|SFI scores for individual animals in different groups.
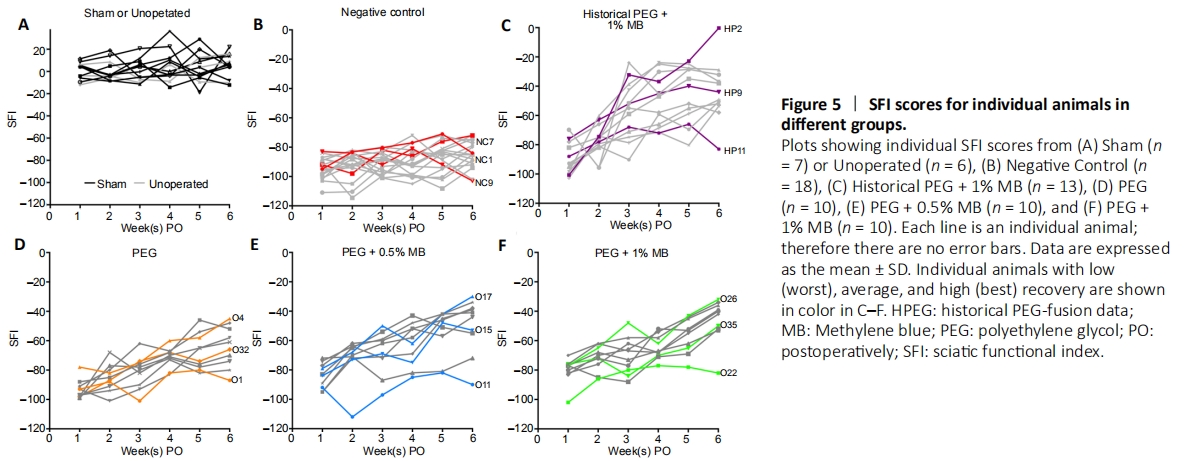
Figure 5 shows the SFI scores for individual animals in all treatment groups. Unoperated and Sham Control animals (Figure 5A) showed no general trend in scores from 1–6 weeks post-operatively. Negative Control animals (each individual animals deisgnated as “NC” followed by assigned number, Figure 5B) all showed loss of function and little, if any, recovery. Historical PEG + 1% MB animals (each individual animal designated as “HP” followed by assigned number, Figure 5C) showed a general trend of increased SFI scores over time. SFI scores for most individual animals in the PEG, PEG + 0.5% MB and PEG + 1% MB experimental groups (each individual animal designated as “O” followed by assigned number, Figure 5D–F) showed a general trend of increased SFI scores over time. However, a few individual animals in the different PEG groups (Figure 5D–F) showed little or no recovery of their SFI scores by 42 days PO. The lowest SFI scores for individual PEG treated animals at 42 days PO in Figure 5D–F (O1; SFI = –87), PEG + 0.5% MB (O11; SFI = –90) and PEG + 1% MB (O22; SFI = –82) were similar to SFI scores at any PO time for the NC group (Figure 5B). Individual PEG treated animals with intermediate SFI recovery at 42 days PO (O32; SFI = –66), PEG + 0.5% MB (O15; SFI = –53) and PEG + 1% MB (O35; SFI = –50) all had higher SFI scores than NC animals at 42 days PO (Figure 5B). Individual PEG-treated animals with the greatest SFI recovery (O4; SFI = –45), PEG + 0.5% MB (O17; SFI = –34) and PEG + 1% MB (O26; SFI = –32) achieved SFI scores never observed for any individual NC animal at any PO time.
Figure 6|Representative footprints of animals from control groups.
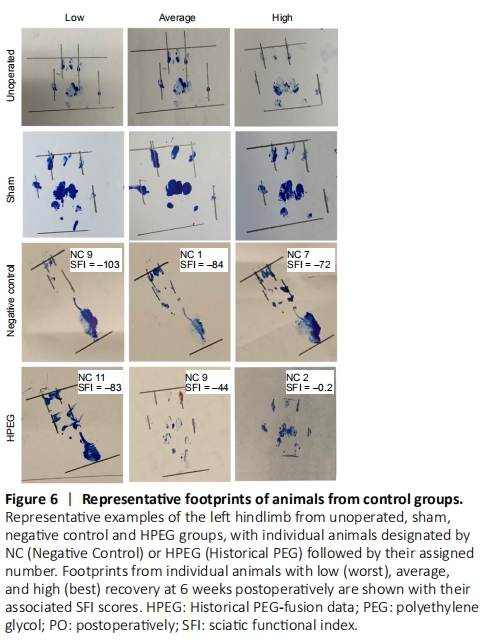
Figure 7|Representative footprints of animals from experimental groups.
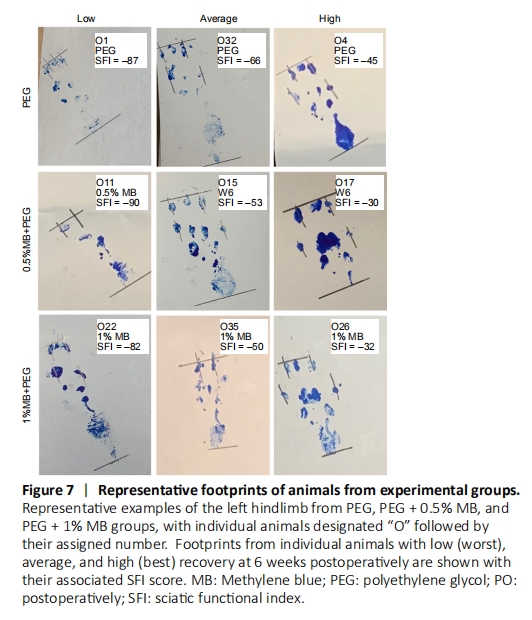
Representative footprints for individual animals in Unop and Sham control groups (Figure 6) showed no obvious difference between the two groups or at any PO time. All animals in these control groups were able to lift their heel and spread toes during locomotion required for the SFI test. In contrast, all NC animals (each individual animal designated as “NC” followed by assigned number) lost the ability to completely lift their heel off the ground, and total toe spread and intermediate toe spread only modestly recovered for individual NC animals with the highest SFI scores (NC7; SFI = –72). Individual NC animals with low SFI scores (NC9; SFI = –103) completely lost sciatic all nerve mediated behaviors. For individual HPEG animals (each individual animal designated as “HPEG” followed by assigned number) with the lowest (worst) SFI score at 42 days PO (HPEG11; SFI = –84) had footprints similar to the average individual NC animal at 42 days PO (NC1; SFI= –84). Footprints for individual HPEG animals with average to excellent SFI scores showed intermediate and total toe spread similar to those of individual Unop or Sham control animals but lacked an ability to completely lift their heel off the ground during locomotion in the SFI test. Individual animals in the PEG, PEG + 0.5% MB and PEG + 1% MB experimental groups (Figure 7) with the worst (lowest) SFI scores exhibited footprints associated with footprints of individual NC animals with similar SFI scores. Individual animals in the PEG-treated groups with average to excellent SFI scores also had well-spread toes similar to those observed for individual Unop and Sham controls and were also unable to completely lift their heel off the ground.