神经损伤与修复
-
Figure 1|Non-human primate models for the preclinical testing of cell replacement therapies.
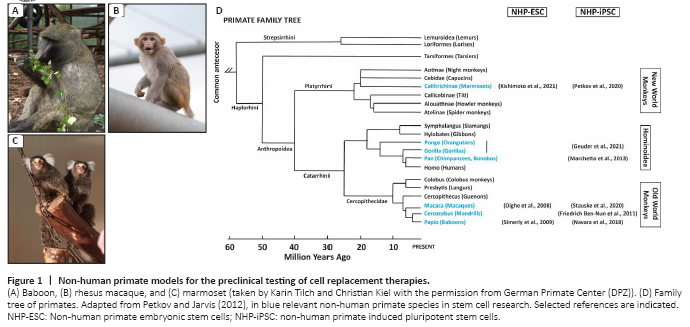
PSC can be differentiated into all cell types of the adult body. Additionally, they are easily expandable, making it possible to generate therapeutically sufficient numbers of differentiated cells in a relatively short time. Further, PSC can be genetically manipulated in order to reduce immunogenicity issues (Shi et al., 2017; Wang et al., 2020). Embryonic stem cells (ESC) are pluripotent cells that are derived from the preimplantation embryo. However, the clinical application of ESC is limited due to ethical controversies associated with their derivation. Furthermore, the use of ESC essentially represents an immunologically non-preferred allograft transplant situation. In contrast, induced pluripotent stem cells (iPSC), derived from somatic cells by reprogramming, circumvent both problems, the ethical issue and the use of allogeneic cells. Takashi and Yamanaka (2006, 2007) discovered a method to induce pluripotency in adult cells and generated the first iPSC lines. Since their discovery, therapies using iPSC as advanced therapy medical products (ATMP) to regenerate tissues and organs have quickly developed and are currently in an advanced state close to translation to the clinics (Nagoshi et al., 2020; Yamanaka, 2020). However, some important points need to be addressed before the final translation of these technologies. There are still open questions regarding the minimal effective dose, route of administration, medium/long-term rejection risks, long-term efficiency, or the feasibility for the implementation of an efficient infrastructure for a broad administration of these therapies (Shi et al., 2017; Liu et al., 2020; Wang et al., 2020; Tao et al., 2021). This needs to be individually evaluated for each disease, organ, therapeutic strategy, and possibly also for the different sexes and ages. Therefore, it is necessary to perform highly predictive studies using preclinical animal models for safe clinical translation. Non-human primates (NHP) are excellent models to test cell replacement therapies due to their close phylogenetic relationship with humans, which is reflected by high genetic, metabolic, and physiological similarities (Johnsen et al., 2012; Cox et al., 2017; Cardoso-Moreira et al., 2020; Figure 1).
Figure 2|Representative bright field pictures of undifferentiated human and non-human primate transgene-free induced pluripotent stem cells (iPSC) colonies in feeder-free conditions.
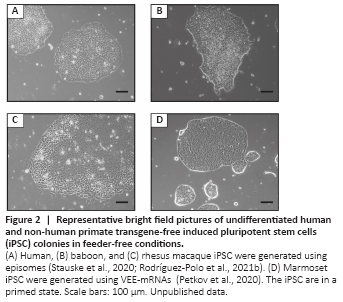
Non-integrative reprogramming approaches: Non-DNA-integrative methods work less efficiently in comparison with integrative methods (Schlaeger et al., 2015). However, these systems allow reprogramming without modification of the genome. Non-integrative DNA methods like episomal vectors provide the advantage of easy amplification of the reprogramming tools and allow generating transgene-free iPSC. Stauske et al. (2020) used episomal vectors (Okita et al., 2011) based on the EBNA sequence to reprogram skin fibroblasts from macaque and baboon (Figure 2A–C). This system has also been successfully used to reprogram marmoset iPSC (Vermilyea et al., 2017; Additional Table 2). The plasmid sequences contain Epstein-Barr virus-derived sequences (EBNA) that allow the episomes to replicate in coordination with the cellular genomic DNA. However, EBNA expression gets progressively silenced during the first passages of the iPSC after reprogramming, leading to the progressive loss of the episomes. DNA-based reprogramming methods, however, even the non-integrating ones, entail the risk of unintended genomic integration. Therefore, RNA-based methods were developed to circumvent the risk of intended or unintended genomic alterations by DNA-based approaches. The use of RNA to reprogram somatic cells discards the possibility of genomic integration. Sendai virus-based vectors can be used to deliver self-replicating reprogramming factor mRNA (Fusaki et al., 2009; Nishimura et al., 2011). Several groups have reported novel, transgene-free NHP-iPSC generated using this system, including baboon (Navara et al., 2018), gorilla, orangutan (Geuder et al., 2021), and macaque (Coppiello et al., 2017). Watanabe et al. (2019) also used an RNA method to reprogram cynomolgus monkey and marmoset cells. They performed serial transfections of mRNA (Watanabe et al., 2019) and followed a workflow developed for human cells, combining reprogramming factor mRNAs (OSKMLN) with ESC‐specific miRNAs (302a‐d, 367) and vaccinia virus‐derived interferon response suppressor mRNAs (E3, K3, and B18R). Petkov et al. (2020, 2021) used a similar reprogramming approach, but in order to avoid the need for serial transfections, self-replicating mRNA vectors based on the Venezuelan equine encephalitis virus (VEE-mRNAs) were used to reprogram marmoset fetal fibroblasts. This method allowed the generation of marmoset iPSC and neural stem cells with a single RNA transfection (Petkov et al., 2020; Petkov and Behr, 2021; Figures 1 and 2D). Alternative to DNA and RNA methods, protein-based reprogramming has been reported for human iPSC generation (Kim et al., 2009; Zhang et al., 2012; Seo et al., 2017). However, this method has low efficiency and, to our knowledge, has not been successfully applied to reprogram NHP cells.
Figure 3|Overview of the workflow for testing pluripotent stem cells (PSC)-based regeneration therapies in non-human primates (NHP).
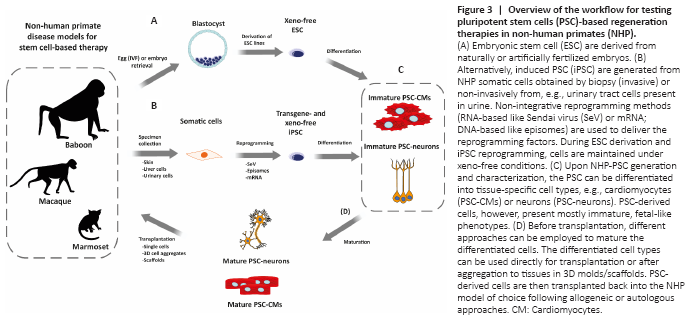
Considering all progress made over the last years in the generation of NHP-iPSC it is possible to say that reprogramming methods for human cells can be translated directly to NHP (Additional Table 2). The state-of-the-art reprogramming methods include, from our point of view, episomes, Sendai virus, RNA vectors, and mRNAs (Figure 3 and Additional Table 2). However, differences in reprogramming efficiency between human and NHP cells have been pointed out by a few studies (Friedrich Ben-Nun et al., 2011; Schlaeger et al., 2015; Stauske et al., 2020). This can theoretically be due, as mentioned above, to the difference between human exogenous and NHP endogenous pluripotency factors. However, the expression of marmoset exogenous factors in marmoset fibroblasts also did not result in high reprogramming efficiency (Debowski et al., 2015). Alternatively, most of the culture media, small molecules, inhibitors, and other supplements used during reprogramming, are designed and optimized for human cells. Additive effects of small differences in the performance of the growth factors and supplements in human cells and cells from other species may be the main reason for the lower reprogramming efficiency seen in many non-human cells. Lastly, unknown endogenous differences between human and NHP cells may account for the observed differences in the respective reprogramming efficiencies.