周围神经损伤
-
Figure 1|Identification of BMSCs and BMSC-Exos.

Optical microscope images demonstrated that BMSCs had spindle morphology (Figure 1A). After induction of differentiation, calcium deposits inside and outside the cells appeared red after alizarin red staining (Figure 1B). Red lipid droplets were found in BMSCs after Oil red O staining (Figure 1C). Figure 1D shows the round morphology typical of chondrocytes. Flow cytometry (Figure 1E) showed that CD29 and CD90 were positive (both > 95%) and CD34 and CD45 were negative (both < 5%). In conclusion, the cells used in our experiments met the characteristics of BMSCs.
TEM analysis showed that the exosomes isolated from the supernatants of BMSCs had a typical lipid bilayer membrane and cup-shaped morphology (Figure 1F). Nanoparticle tracking analysis revealed that the exosomes were 110.3 ± 2.6 nm in diameter (Figure 1G). The specific exosome markers CD9, CD63, and TSG101 were positive (Figure 1H).Figure 2|Morphology and release kinetics of the exosomes from different conduits.

Chi conduits were nearly transparent, while the Chi/PDA conduits had low transparency and appeared black (Figure 2A and B). Chi-Exos and Chi/PDA-Exos conduits were constructed by immersing Chi and Chi/PDA conduits in BMSC-Exos solution (Figure 2C). A scanning electron microscope was used to observe the number and morphology of exosomes on the surface of Chi and Chi/PDA conduits. The Chi conduit was composed of many disordered micron-scale Chi fibers (Figure 2D). More BMSC-Exos were distributed on the surface of Chi/PDA conduits than Chi conduits (P < 0.01; Figure 2E and F). The exosome-loading qualities of Chi and Chi/PDA conduits were 346.7 ± 8.82 μg and 710 ± 17.32 μg, respectively (Figure 2G). Furthermore, the sustained-release experiment demonstrated that exosomes were burst released from Chi conduits in the first 48 hours, and few exosomes were detected after 10 days. In contrast, there was a pattern of sustained release of immobilized exosomes from Chi/PDA conduits within 14 days. Approximately 22.56 ± 2.42% of the exosomes remained in the Chi/PDA conduits after 14 days (Figure 2H and I).
Figure 3|Chi/PDA-Exos provide more exosomes than Chi-Exos.

On the 1st day after treatment, we found that the area of PKH26-labeled BMSC-Exos in the phalloidin-labeled SCs of the Chi-Exos group was larger than that of the Chi/PDA-Exos group (P < 0.01). On the 5th day after treatment, the area of PKH26-labeled BMSC-Exos was smaller in the Chi-Exos group than in the Chi/PDA-Exos group (P < 0.01; Figure 3A and B).
Figure 4|Chi/PDA-Exos improve SC proliferation and secretory capacity and activate SC reprogramming.
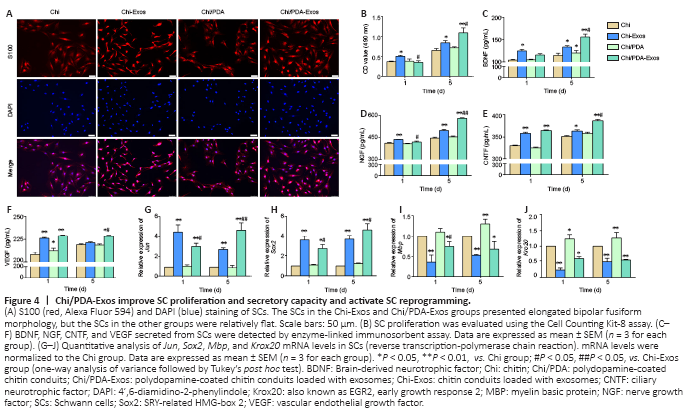
On the 5th day after treatment, the SCs in the Chi-Exos and Chi/PDA-Exos groups had elongated bipolar fusiform morphology, which is consistent with the typical morphology of repaired SCs, but the SCs in the other groups were comparatively flat (Figure 4A). CCK-8 results showed proliferation of SCs was highest in the Chi-Exos group 1 day after treatment (P < 0.05, vs. Chi group) (Figure 4B). On the 5th day after treatment, Chi/PDA-Exos gave an obvious advantage in promoting SC proliferation (P < 0.01, vs. Chi-Exos group) (Figure 4B).
The protein expression of three neurotrophic factors (nerve growth factor, brain-derived neurotrophic factor, and ciliary neurotrophic factor) and vascular endothelial growth factor was analyzed by ELISA. On the 1st day after treatment, we found that SCs in the Chi-Exos group secreted higher levels of neurotrophic factors and vascular endothelial growth factor than SCs in the other three groups (P < 0.05). Chi/PDA-Exos gave excellent regulation of SC secretory function 5 days after treatment (Figure 4C–F).
The expression levels of the repair-phenotype marker genes (Jun and Sox2) and myelin-related genes (Mbp and Krox20) were evaluated by reverse transcription-polymerase chain reaction. No significant difference was found in the genes’ expression levels in SCs treated with Chi or Chi/PDA (P > 0.05), while exosome treatment upregulated the mRNA expression levels of Jun and Sox2 and downregulated the mRNA expression levels of Mbp and Krox20 on the 1st day after treatment (P < 0.05). PDA also increased the mRNA expression of Mbp and Krox20 on the 5th day after treatment. The sustained release of exosomes continued to activate the SC repair phenotype (P < 0.05; Figure 4G–J).Figure 5|Chi/PDA-Exos enhances neurite growth of DRGs after 7 days of treatment.

Immunofluorescence staining was performed to evaluate the effect of Chi/PDA-Exos on DRG neurites (Figure 5A). On the 7th day, the average length of DRG neurites treated with Chi-Exos, Chi/PDA, or Chi/PDA-Exos was significantly increased compared with the Chi group (P < 0.01; Figure 5B).
Figure 6|Chi/PDA-Exos improve functional recovery and gastrocnemius muscle morphometry in rats with sciatic nerve injury.

Rat footprints and the SFI rating scale were used to evaluate functional recovery of PNI up to 8 weeks after surgery. Results revealed unambiguous footprints with better toe spread in the Chi/PDA-Exos group compared with other groups (Figure 6A). The SFI value of the Chi/PDA-Exos group was clearly superior to the Chi and Chi-Exos groups 8 weeks postoperation (P < 0.01; Figure 6B).
Eight weeks after surgery, sciatic nerve conduction of all groups was examined. The CMAP latency of the Chi/PDA-Exos group was lower than the Chi group (P < 0.01) and was similar to the Chi-Exos group (P > 0.01). The CMAP amplitude of the Chi/PDA-Exos group was higher than the Chi and Chi-Exos groups (P < 0.01; Figure 6C–E).
Eight weeks after surgery, we observed different degrees of atrophy of the gastrocnemius muscle on the operated side (Figure 6F). The wet weight rate of the gastrocnemius muscle in the Chi/PDA-Exos group was significantly higher than in the Chi and Chi-Exos groups (P < 0.05; Figure 6G). As shown in Figure 6H and I, Masson’s trichrome staining showed that the cross-sectional area of the muscle fiber in the Chi/PDA-Exos group was larger than in the Chi and Chi-Exos groups (P < 0.05).Figure 7|Chi/PDA-Exos improve the recovery of regenerated nerve fibers in rats with sciatic nerve injury 8 weeks after implantation.
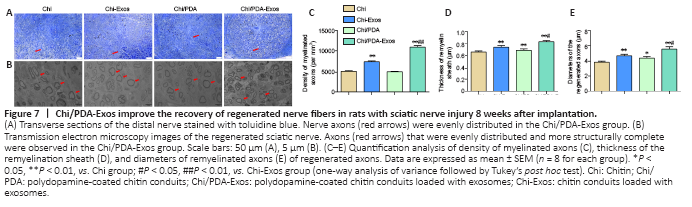
Eight weeks after surgery, the regenerated sciatic nerve was exposed and evaluated. SC myelination plays a critical role in axonal regeneration after PNI (Nocera and Jacob, 2020). Toluidine blue staining was performed 8 weeks post-implantation (Figure 7A). TEM was performed to evaluate the myelin sheath thickness and the diameter of regenerated myelinated nerve fibers (Figure 7B). Compared with the Chi group, the numbers of axons at the distal ends of the regenerating nerve bundles were increased significantly in the Chi-Exos and Chi/PDA groups, and especially in the Chi/PDA-Exos group (Figure 7C). TEM showed that the laminated myelin sheaths of regenerated nerve fibers in the Chi-Exos and Chi/PDA groups were greater than in the Chi group (P < 0.05). We also found the mean diameter of regenerated axons in the Chi/PDA-Exos group was the largest compared with the other groups (Figure 7D–E).