周围神经损伤
-
Figure 1|Bioactive hydrogel (VEGF@GelMA) accelerates peripheral nerve regeneration after crush injury.
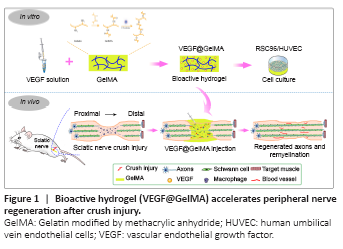
In this study, we hypothesized that administration of VEGF-laden GelMA hydrogel directly into a crushed sciatic nerve would promote its functional recovery, reinnervation and vascularization. To confirm this, VEGF165 (a VEGF-A isoform) was incorporated into GelMA, to form VEGF@GelMA hydrogel, to achieve a controlled release of this multifunctional growth factor. Initially, the compatibility of the hydrogel and bioactivity of the hydrogel leachate were investigated in vitro. Finally, the neurogenesis and angiogenesis capacities of this VEGF-laden GelMA hydrogel were evaluated in an established crush injury model of rat sciatic nerves (Figure 1).
Figure 2|VEGF@GelMA hydrogel characterization and fabrication.
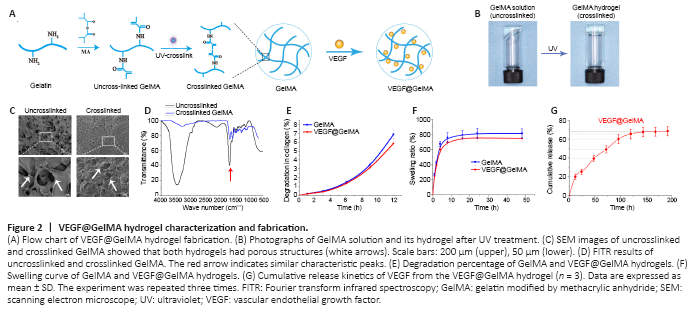
The fabrication process of the composite hydrogel is shown in Figure 2A. After irradiation for 20 seconds by ultraviolet light, the uncrosslinked GelMA solution formed a GelMA composite hydrogel (Figure 2B). The uncrosslinked solution had good fluidity, which meant that it could be directly administered to the injured site and then form a bioactive cumulative-releasing hydrogel by UV irradiation. After freeze drying, we observed the microstructures of both uncrosslinked and crosslinked GelMA. Both had a porous-connected structure (Figure 2C), which is advantageous for cell adhesion and nutrient exchange. The Fourier transform infrared spectroscopy spectrum of uncrosslinked GelMA was similar to that of crosslinked GelMA (Figure 2D). The degeneration and swelling properties of the GelMA hydrogel and VEGF@GelMA hydrogel were also evaluated. As shown in Figure 2E, the degeneration rate of the VEGF@GelMA hydrogel in collagenase-2 solution was slightly lower than that of the GelMA hydrogel. The swelling ratios of these two hydrogels were very similar (Figure 2F), which indicates that the incorporation of VEGF did not influence the capacity of the GelMA hydrogel to absorb water. As shown in Figure 2G, a sustained release of VEGF could be achieved with the VEGF@GelMA hydrogel, avoiding a burst release of VEGF and facilitating its subsequent in vitro or in vivo applications.
Figure 3|Biocompatibility of the VEGF@GelMA hydrogel detected by RSC96 cells and HUVECs.
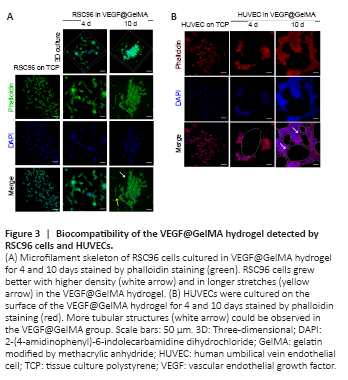
Cell adhesion, cell morphology and growth status were the basic parameters for assessing the biocompatibility of the hydrogel (Liu et al., 2021). Initially, we cultured RSC96 cells in the VEGF@GelMA hydrogel using a three-dimensional culture. After culturing for 10 days, the RSC96 cells grew more with higher density and longer stretches in the VEGF@GelMA hydrogel than on tissue culture polystyrene (TCP), as indicated by phalloidin staining (Figure 3A). To further evaluate the bioactivity of the composite hydrogel, HUVECs were seeded on the surface of the hydrogel. As shown in Figure 3B, compared with TCP group, many more tubular structures could be observed in the VEGF@GelMA group, which suggested a proangiogenic effect of the bioactive hydrogel in vitro.
Figure 4|Establishment of the sciatic nerve crush injury rat model.
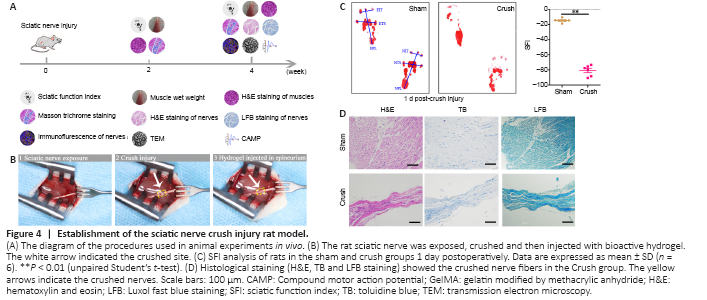
The chronological diagram of in vivo animal experiments is shown in Figure 4A. To test the bioactivity of the VEGF@GelMA hydrogel in vivo, we first established the rat sciatic nerve crush injury model, which is widely used to study the regeneration of the PNS (Varej?o et al., 2004; Lee et al., 2020). Briefly, crush injury with hemostatic forceps broke down the internal axons and surrounding myelin sheaths but preserved the epineurium and basal lamina. Then, PBS or the hydrogel was directly administered intra-epineurium to the crushed nerve and surrounding tissues with a microsyringe (Figure 4B). The crush effects were confirmed through functional and histological analysis. After the nerve crush, the SFI score decreased (P < 0.01; Figure 4C). The histological staining (H&E, toluidine blue and Luxol fast blue) results showed that the distance between the epineurium in the crushed group was considerably narrower than that in the normal sciatic nerve group (Figure 4D), indicating a successful establishment of the rat sciatic nerve crush injury model.
Figure 5|Functional recovery of crushed sciatic nerves after VEGF@GelMA injection at 2 and 4 weeks after sciatic nerve injury.

The in vitro data demonstrated the good biocompatibility and excellent bioactivity of the VEGF@GelMA hydrogel, but in vivo studies were necessary to comprehensively evaluate the outcomes of the composite hydrogel regarding PNS repair. Two and four weeks after surgery, the SFI score was considerably higher in the crush + VEGF@GelMA group than in both the crush + PBS and crush + GelMA groups (both P < 0.05; Figure 5A and B). Target muscle analysis showed that the wet ratios of the gastrocnemius muscle, soleus muscle and anterior tibialis muscle in the crush + VEGF@GelMA group were considerably higher compared with those in the other two groups (P < 0.05; Figure 5C and D). This result indicated that the sustained delivery of VEGF reduced the atrophy of the three target muscles that were innervated by the injured sciatic nerve. H&E and Masson trichrome staining were used for histological analyses of the effect of VEGF on denervated muscular atrophy (Figure 5E–H). Four weeks postoperatively, the cross-sectional area of muscle fibers was significantly higher in the crush + VEGF@GelMA group than in the crush + PBS (P < 0.01) and crush + GelMA (P < 0.05) groups (Figure 5F). However, the average percentage of collagen fiber area in the crush + VEGF@GelMA group was more pronounced than those in the crush + GelMA (P < 0.01) and crush + PBS groups (P < 0.05; Figure 5H). All these data indicated that the denervation of target muscles and muscle atrophy resulting from crush injury could be attenuated by VEGF@GelMA hydrogel injection therapy.
Figure 6|Histological analysis of sciatic nerves 4 weeks after hydrogel injection in vivo.
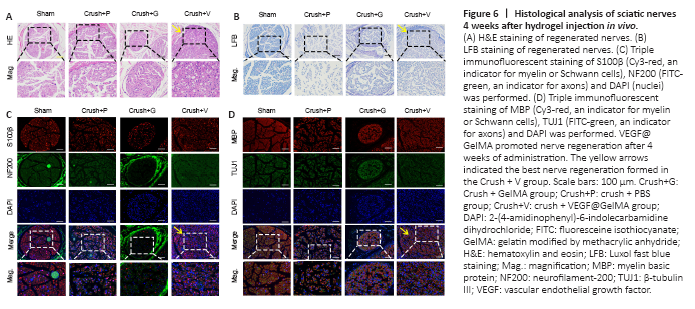
Nerve repair 4 weeks post crush injury was determined through histological and electrophysiological examinations. Representative images of H&E staining and Luxol fast blue staining of the four groups are displayed in Figure 6A and B. These results indicated that more axonal regeneration and remyelination developed in the crush + VEGF@GelMA group compared with the other crush groups, however, the nerve fibers were better organized in the sham group. Furthermore, we evaluated axonal regrowth and nerve remyelination through immunofluorescence assays using four critical markers, S100β, neurofilament-200, beta III Tubulin and myelin basic protein. Neurofilament-200 and beta III Tubulin indicated regenerated axons and neurofilaments, whereas the expression of S100β and myelin basic protein are measures of axonal remyelination status (Cattin et al., 2015; Fan et al., 2018). Figure 6C and D show that the levels of these indicators were stronger in the crush + VEGF@GelMA group than those in the crush + PBS and crush + GelMA groups. These data suggested that VEGF@GelMA promoted nerve regeneration after 4 weeks of administration.
Figure 7|Crushed sciatic nerves show differential myelination and electrophysiological performance after hydrogel administration.
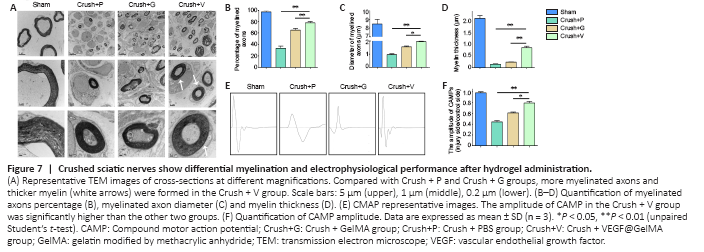
TEM was used to evaluate the axons and their remyelination of regenerated nerve fibers. Representative images of the middle segment of each nerve sample are displayed in Figure 7A. At 4 weeks post-injury, some myelinated axons had developed in the crush + PBS groups that suggested Wallerian degeneration of the sciatic nerve in contrast to the uniform structure bounded by electron-dense, clear and thick myelin sheaths observed in the sham group. Quantitative analysis parameters (including myelin thickness, myelinated axons diameter and myelinated axons percentage) were also used to assess regenerative efficacy. All values of the above three parameters were highest in the crush + VEGF@GelMA group compared with the values in the crush + PBS and crush + GelMA groups (P < 0.05 or P < 0.01; Figure 7B–D). Electrophysiology of neurons accurately quantifies nerve function (Lu et al., 2019). In our study, the compound motor action potential amplitude in the crush + VEGF@GelMA group was higher than in either the crush + PBS (P < 0.01) or crush + GelMA groups (P < 0.05; Figure 7E and F), also indicating that repair of the crushed nerve is accelerated when using the bioactive hydrogel.
Figure 8|VEGF@GelMA hydrogel promotes angiogenesis of regenerated nerves.

In tandem with our evaluations of neuronal regeneration, we studied vascularization improvement after VEGF@GelMA hydrogel injection into the injury site. CD31 is widely involved in the angiogenesis process and may act through modulating the intercellular junctions of endothelial cells (Cattin et al., 2015). The results of CD31 immunofluorescence staining suggested significantly more formation of microvessels in the crush + VEGF@GelMA group compared with the other three groups (P < 0.05; Figure 8A and B). The presence of α-SMA indicates that the neovascular tissues progress become into mature vessels (Guan et al., 2021). The α-SMA-positive mature vessel density was also considerably higher in the crush + VEGF@GelMA group compared with either the crush + PBS or crush + GelMA groups (P < 0.05; Figure 8A and C), which indicated that VEGF delivery promoted vessel maturation. Consistent with this, the protein expression of CD34 and von Willebrand factor (important factors associated with vascular tissues) (Abdulkadir et al., 2020) measured by western blot further confirmed the proangiogenic effects of the VEGF@GelMA hydrogel (Figure 8D and E). These data showed that controlled release of VEGF from the bioactive hydrogel promoted vascularization, which may provide nutrients, remove waste and facilitate nerve regeneration synergistically.