周围神经损伤
-
Figure 2| Identification of BMSCs.
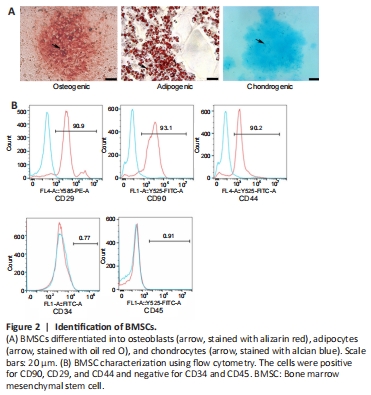
The multidirectional differentiation ability of BMSCs was examined, including adipogenic differentiation by oil red O staining, osteogenic differentiation by alizarin red S staining, and chondrogenic differentiation by alcian blue staining (Figure 2A). The results showed that the BMSCs were able to differentiate into adipocytes, osteocytes, and chondrocytes. The BMSC phenotypes were examined by flow cytometry (Figure 2B). The cells were positive for the mesenchymal stem cell markers CD29, CD90, and CD44 and negative for CD34 and CD45, which demonstrated that the cells extracted from the rat bone marrow were BMSCs.
Figure 3|Identification of BMSC-derived EVs and effect of the EVs on tube formation by human umbilical vein endothelial cells (HUVECs).
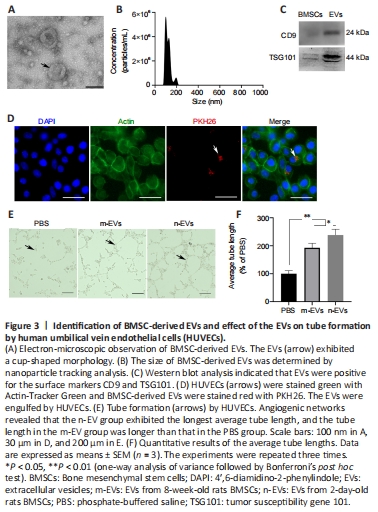
BMSC-derived EVs were isolated from the cell supernatants with a final concentration of 1135 μg/mL. The EVs were observed by transmission electron microscopy and showed a typical cup-shaped morphology (Figure 3A). Nanoparticle tracking analysis revealed that the diameters of the EVs mainly ranged from 30–200 nm (Figure 3B). Expression of the EV surface markers CD9 and TSG101 was positive by western blot (Figure 3C). We characterized the particles by their appearance, particle size, and surface markers, and the isolated particles demonstrated the features of EVs.
To examine the uptake of BMSC-derived EVs in vitro, we labeled the EVs and tracked them successfully after engulfment by HUVECs. After a 24-hour coculture with HUVECs, Figure 3D clearly shows that EVs were present within the cytoplasm of the HUVECs. Tube formation assays were used to determine the pro-angiogenic effect of BMSC-derived EVs. The assays showed that the addition of EVs promoted an increase in tube lengths, and the EVs from 2-day-old rat BMSCs performed significantly better than EVs from 8-week-old rat BMSCs (Figure 3E and F).
Figure 4|Effect of loading rGO-GelMA-PCL nerve conduits with BMSC-derived EVs on sciatic nerve functional recovery in rats with nerve defects at 12 weeks after surgery.

The electrical conduction of regenerated nerves and the reinnervation of the muscles were detected using electrophysiological methods (Figure 4A). The amplitude of compound muscle action potential in the EV group was higher than that in the control group, which indicated that the addition of EVs improved muscle reinnervation; however, the autograft group displayed a higher amplitude than both the control and EV groups (Figure 4B). The motor nerve conduction velocity was higher in the EV group than that in the control group, which indicated that the addition of EVs improved myelin sheath recovery, and the velocity in the EV group was not significantly different from that in the autograft group (Figure 4C).
Figure 6|Effect of loading rGO-GelMA-PCL nerve conduits with BMSC-derived EVs on regenerated nerve microstructure in rats with nerve defects.
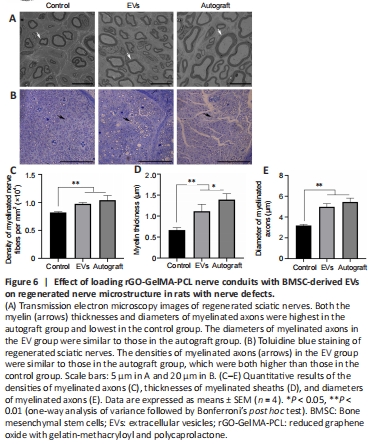
The ultra-structure of the regenerated nerve was evaluated by transmission electron microscopy (Figure 6A), the myelin sheaths were stained with toluidine blue (Figure 6B), and the gross numbers of the regenerated nerve fibers were counted (Figure 6C). Cross sections of the middle part of the regenerated nerve showed that the nerve fiber density for each group was different. The density of myelinated nerve fibers in the EV group was higher than that in the control group, and no difference was observed between the EV and autograft groups (P > 0.05).
In Figure 6A, each regenerated axon was covered with a myelin sheath, although sheath thickness differed among the groups. The regenerated sheaths in the EV group were thicker than those in the control group and similar to those in the autograft group (P > 0.05), which illustrated the positive effect of BMSC-derived EVs on Schwann cells surrounding the nerve fibers. Moreover, the diameter of myelinated axons in the EV group was also higher than that in the control group, and there was no significant difference between the EV and autograft groups (Figure 6D and E).Figure 7|rGO-GelMA-PCL nerve conduits loaded with BMSC-derived EVs promotes the regeneration of sciatic nerves at 12 weeks after surgery.

Double NF200/S100 immunofluorescent staining of the middle part of the grafts showed that both axons and myelin sheaths had migrated from the proximal nerve stump to the distal nerve stump at 12 weeks after surgery. The percentages of NF200- and S100-positive areas in the EV group were demonstrably higher than those in the control group, and the percentages of S100-positive areas were similar for the EV and autograft groups (P > 0.05; Figure 7).
Figure 8|rGO-GelMA-PCL nerve conduits loaded with BMSC-derived EVs improve angiogenesis in rats with nerve defects at 12 weeks after surgery.
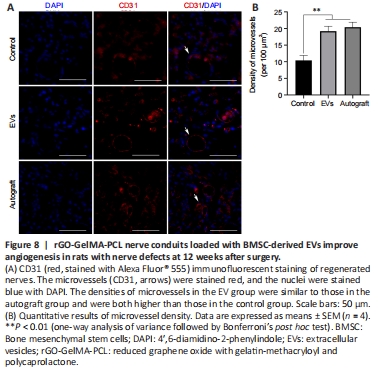
Angiogenesis plays an important role in the process of tissue regeneration (Gon?alves et al., 2021). Statistical analysis showed that microvessel density in the EV group was significantly higher than that in the control group (P < 0.01) and was similar to that in the autograft group (P > 0.05; Figure 8).