神经退行性病
-
Figure 2|Pretreatment with an anti-IGF-2 neutralizing antibody reduces the neuroprotective effect of SKP-SCs-CM on 6-OHDA-treated SH-SY5Y cells.
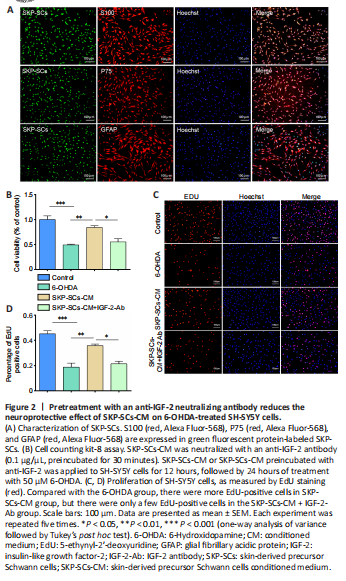
We recently demonstrated the neuroprotective effect of SKP-SCs (Chen et al., 2020; Ma et al., 2021; Yan et al., 2022). Conditioned medium from SKP-SCs can reduce the injury that 6-OHDA causes to SH-SY5Y cells, and direct transplantation of SKP-SCs can reduce 6-OHDA-induced dopaminergic neuron damage in vivo and in vitro by regulating autophagy. In our previous studies, we analyzed SKP-SCs-CM by mass spectrometry and identified the cytokine IGF-2 as being potentially related to the therapeutic effects of SKP-SCs. This factor is a member of the insulin polypeptide family, which is expressed at high levels in the brain and plays an instrumental role in brain development. In this study, we cultured SKP-SCs and confirmed their identity by immunofluorescence staining for the Schwann cell markers S100, P75, and GFAP (Figure 2A).
Next, we explored whether IGF-2 is involved in the neuroprotection mediated by SKP-SCs-CM. An IGF-2 neutralizing antibody was added to SKP-SCs-CM, which were then pre-incubated at 37°C for 30 minutes. Both the CCK-8 and the EdU results showed that the neuroprotective effect of SKP-SCs-CM was eliminated by pre-incubation with the neutralizing antibody. As shown in Figure 2B, compared with the 6-OHDA injury group, the SKP-SCs-CM pretreatment group exhibited significantly greater cell viability (P < 0.01). However, SKP-SCs-CM pre-incubated with the anti-IGF-2 neutralizing antibody was unable to protect SH-SY5Y cells from the damage induced by 6-OHDA. The EdU results were consistent with the CCK-8 results (Figure 2C and D). These results suggest that IGF-2 plays a crucial role in SKP-SCs-CM–mediated neuroprotection.
Figure 4|IGF-2 pretreatment reduces the damage to SH-SY5Y cells induced by 6-OHDA.
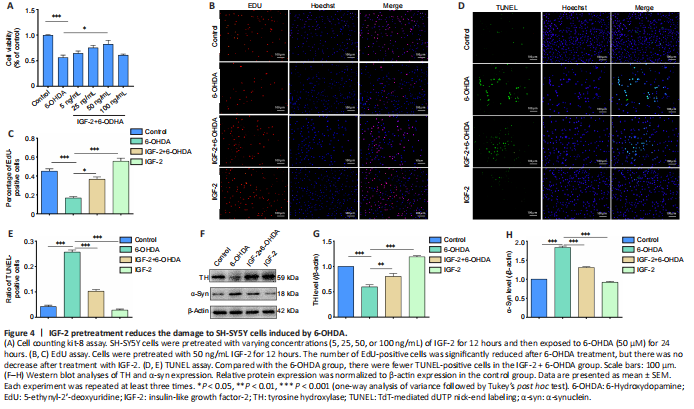
To determine whether IGF-2 is responsible for the observed neuroprotection, we pre-treated 6-OHDA-induced SH-SY5Y cells with recombinant IGF-2 at different concentrations (5, 25, 50, 100 ng/mL) and then measured cell viability by CCK-8 assay (Figure 4A). The results showed that IGF-2, when administered in a certain concentration range, inhibited the 6-OHDA-induced cytotoxicity. In particular, 50 ng/mL IGF-2 had a significant protective effect (P < 0.05, vs. 6-OHDA group).
Based on the CCK-8 results described above, we chose 50 ng/mL IGF-2 as the maximum drug concentration for subsequent experiments. Next, we tested cell proliferation by EdU assay. As illustrated in Figure 4B and C, the number of proliferating cells was significantly reduced after 6-OHDA treatment (P < 0.001, vs. control group), but there was no significant decrease in proliferation as a result of treatment with IGF-2. We then performed a TUNEL test to further verify the ability of IGF-2 to rescue 6-OHDA-induced cytotoxicity. Compared with the 6-OHDA group, there were fewer TUNEL-positive cells in the IGF-2 + 6-OHDA group (P < 0.001; Figure 4D and E).
Finally, we performed western blot analysis to determine the protein expression levels of TH and α-syn. The results showed that 6-OHDA treatment significantly decreased TH levels and increased α-syn levels (P < 0.001, vs. control group). Pretreatment with IGF-2 prevented the reduction in TH levels (P < 0.01, vs. 6-OHDA group) and reduced α-syn aggregation (P < 0.001, vs. 6-OHDA group; Figure 4F–H). In summary, IGF-2 has a protective effect in an in vitro 6-OHDA-induced model of PD.
Figure 5|Effect of intranasal IGF-2 administration on 6-OHDA-induced injury in mice.
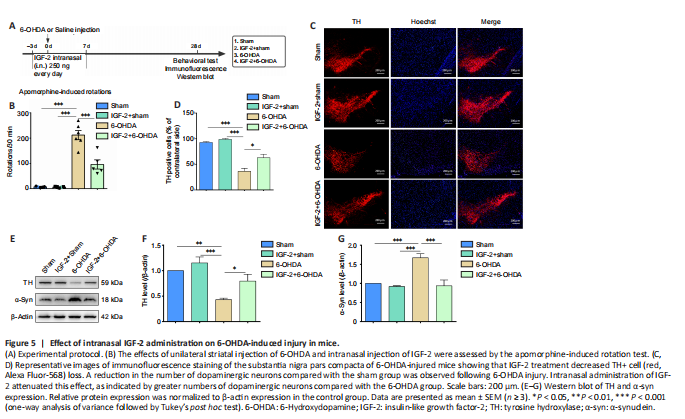
Next, we conducted in vivo experiments to verify our in vitro results. We treated C57BL/6J mice with 6-OHDA to mimic PD, administered IGF-2 intranasally, and performed an apomorphine-induced rotation experiment to evaluate the success of the model (Figure 5A). Twenty-eight days after 6-OHDA injection, the number of contralateral rotations had increased significantly (P < 0.001, vs. sham group), suggesting that 6-OHDA had damaged the dopaminergic system. Treatment with IGF-2 reduced the number of rotations induced by apomorphine (P < 0.001, vs. 6-OHDA group, Figure 5B). Next, the number of TH+ neurons in the substantia nigra was detected by immunofluorescence. In mice that had sustained injury with 6-OHDA, the number of TH+ neurons was significantly reduced (P < 0.001, vs. sham group). Administration of IGF-2 prevent the loss of TH+ neurons (P < 0.05, vs. 6-OHDA group, Figure 5C and D). Finally, we analyzed TH and α-syn protein levels. Consistent with the in vitro results, TH protein expression was significantly decreased after 6-OHDA treatment (P < 0.01), while α-syn protein expression was significantly increased (P < 0.001), and treatment with IGF-2 reversed these effects (Figure 5E–G). These findings suggest that IGF-2 has a neuroprotective effect in vivo.
Figure 6|Double immunofluorescence staining for IGF-2 in the midbrain region of a mouse model of PD.

To explore the specific mechanism by which IGF-2 exerts its neuroprotective effects, we first detected its localization in mouse midbrains by immunofluorescence double-label staining and showed that it was expressed in TH-positive cells, Tuj1-positive neurons, and GFAP-positive neurons (Figure 6). In addition, we measured IGF-2 protein expression levels. Both in vivo (Figure 7A and B) and in vitro (Figure 7C and D) experimental results indicated that IGF-2 expression was reduced after 6-OHDA treatment (P < 0.001, vs. sham group; P < 0.01, vs. control group). Considering that IGF-2 generally acts by binding to its receptors to transduce signals (Hawkes et al., 2006), we next asked whether IGF-2R expression changed during PD development. To test this, we performed western blot analysis of IGF-2R protein expression levels (Figure 7E–J) and found that, in the in vivo experiments, IGF-2R expression was dramatically reduced (P < 0.01, vs. sham group; Figure 7E and H). In parallel, we found in the in vitro experiments that the effect of 6-OHDA treatment on IGF-2R protein expression is time-dependent. As the duration of 6-OHDA injury increased, IGF-2R expression level gradually decreased (P < 0.05, vs. control group; Figure 7F and I). Next, we assessed the effect of treatment with IGF-2 on IGF-2R expression. As can be seen from Figure 7G and J, IGF-2 treatment prevented the 6-OHDA-induced down-regulation of IGF-2R in a time-dependent manner (P < 0.05, vs. 0 h group). The above results suggest that IGF-2 can inhibit 6-OHDA-induced neuronal damage in combination with IGF-2R. Given our previous observation regarding the increase in peripheral serum IGF-2 levels, we speculate that the lack of IGF-2 and its receptor in brain tissue may increase negative feedback increase in the periphery.