脊髓损伤
-
Figure 2|PARP14 is upregulated in spinal cord tissues after SCI, and PARP14 deficiency exacerbates motor dysfunction in a mouse model of spinal cord injury.

GEO database (GSE5296 and GSE52763) analysis showed that PARP14 expression was markedly increased at sites of spinal cord 1, 3, 7, 21, and 28 days after SCI (Additional Figure 1). To explore the role of PARP14 in SCI, we first established the mouse model of SCI. The locomotor function of the mice in each group was analyzed by determining the BMS score. The results showed that mice with SCI had lower scores than the sham mice (Figure 2A), suggesting that the SCI mouse model was established successfully. PARP14 expression in the spinal cord was detected by qPCR, western blot analysis, and immunohistochemistry staining at 1, 7, 14, and 28 days after injury. PARP14 protein and mRNA expression were markedly increased in mice with SCI compared with those in the sham group (Figure 2B–D). Moreover, immunofluorescence double-staining for PARP14 and Iba1 (a microglia marker) showed that compared with the sham group, PARP14 expression was also increased in microglia after SCI (Figure 2E). Next, we knocked down PARP14 expression in the spinal cord by injecting PARP14 short hairpin RNA-carrying lentivirus (Lv-shPARP14) or NC (Lv-shNC) approximately 1 mm above and below the injury site. Our results showed that Lv-shPARP14 injection significantly decreased PARP14 protein expression in the spinal cord 7 days after SCI (Figure 2F). The behavioral assessment demonstrated that administration of LV-shPARP14 started to enhance motor dysfunction 3 days after injection, compared with mice receiving the LV-shNC, and the accelerated progression of motor dysfunction persisted until the final observation at 28 days post-injury (Figure 2G). Footprint analysis (28 days post-SCI) showed that mice with SCI had uncoordinated gaits and exhibited extensive drag of both hindlimbs, and silencing PARP14 aggravated these behaviors (Figure 2H), indicating that PARP14 may play a compensatory role in SCI.
Figure 3| PARP14 deficiency exacerbates SCI-induced neuronal apoptosis at 7 days post-SCI.

Next, we explored the role of PARP14 in neuronal apoptosis. Nissl staining for gray ventral motor neurons showed that mice 7 days post-SCI had more neuron loss than that in the sham mice, and PARP14 knockdown further enhanced SCI-induced neuron loss (Figure 3A). TUNEL and NeuN (a neuronal marker) co-staining showed that compared with the sham group, mice 7 days post-SCI exhibited increased neuronal apoptosis, which was enhanced by PARP14 downregulation (Figure 3B and C). Western blot analysis of apoptosis-related factors showed that cleaved caspase 3 and Bax (pro-apoptotic factors) levels were elevated in the spinal cord of SCI mice, whereas Bcl-2 and Bcl-xl (anti-apoptosis factors) levels were markedly decreased, and PARP14 silencing aggravated both of these trends (Figure 3D).
Figure 4|PARP14 deficiency exacerbates the shift of M2-polarized microglia to M1-polarized microglia in mice 7 days post-SCI.
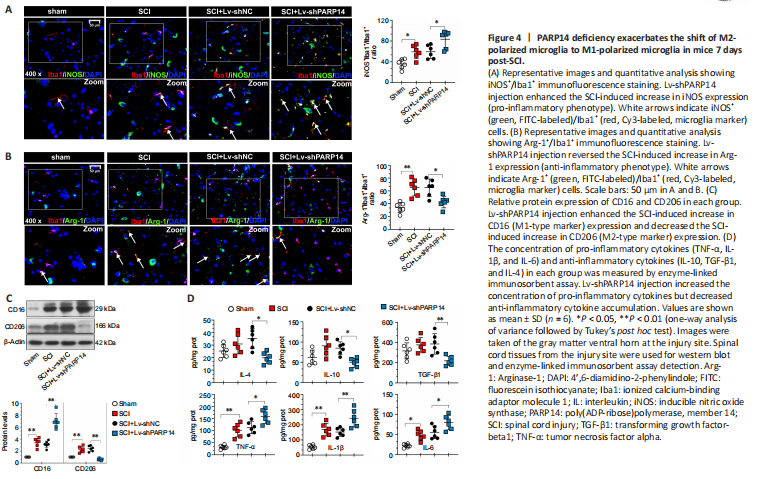
The microglia activation-mediated neuroinflammatory response is an important contributing factor to secondary injury in SCI. Moreover, PARP14 has been reported to regulate macrophage polarization (Iwata et al., 2016). Thus, we explored whether PARP14 regulates microglia polarization in SCI. Double immunofluorescent staining was performed for Iba1 (microglia marker) and M1 (pro-inflammatory phenotype)-associated iNOS or M2 (anti-inflammatory phenotype)-associated Arg-1 (Lisi et al., 2017). The results showed that iNOS and Arg-1 expression were increased in the microglia of mice 7 days post-SCI, and that PARP14 silencing increased iNOS expression but decreased Arg-1 expression (Figure 4A and B). Moreover, western blot analysis indicated that M1-related factor CD16 and M2-related factor CD206 protein levels in the spinal cord of mice 7 days post-SCI were increased, and that PARP14 downregulation significantly increased CD16 expression but decreased CD206 expression (Figure 4C). In addition, PARP14 knockdown promoted the secretion of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and reduced the levels of anti-inflammatory cytokines (IL-10, TGF-β1, and IL-4) in mice 7 days post-SCI (Figure 4D).
Figure 5|PARP14 deficiency activates the STAT1 pathway but blocks the STAT6 pathway in mice 7 days post-SCI.
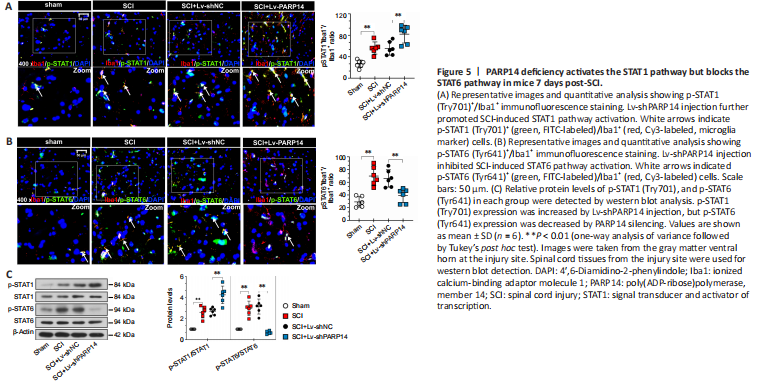
The STAT1 pathway mediates M1 microglia/macrophage polarization, and the STAT6 pathway is a key signaling pathway for M2-like polarization of microglia/macrophages (Gan et al., 2017; Li et al., 2021). Therefore, we next analyzed the effects of PARP14 on the STAT1/STAT6 pathway. Double immunofluorescent staining was performed for Iba1 and p-STAT1 (Try701) or p-STAT6 (Tyr641). When PARP14 expression was knocked down, p-STAT1 expression was increased, whereas p-STAT6 was decreased in mice 7 days post-SCI (Figure 5A and B). Consistent with this, p-STAT1 and p-STAT6 protein levels in the spinal cord of mice 7 days post-SCI were increased, while PARP14 inhibition increased p-STAT1 expression but decreased p-STAT6 expression (Figure 5C).
Figure 6|PARP14 deficiency promotes microglia M1 polarization in vitro.
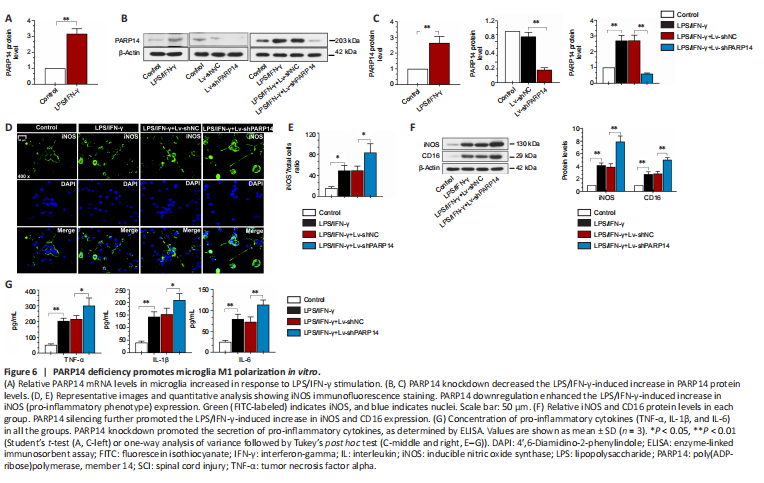
To investigate the function of PARP14 in microglia-mediated neuroinflammatory responses, microglia were treated with LPS/IFN-γ to induce M1 polarization. PARP14 mRNA and protein levels were significantly upregulated in microglia in response to stimulation with LPS/IFN-γ (Figure 6A–C). To silence PARP14 expression in microglia stimulated with LPS/IFN-γ, microglia were transfected with Lv-shPARP14 or Lv-shNC, and the transfection efficiency was verified by western blot analysis (Figure 6B and C). Transfection of microglia stimulated with LPS/IFN-γ with Lv-shPARP14 significantly reduced PARP14 expression (Figure 6B and C). Immunofluorescence staining and western blot analysis showed that the expression of an M1 phenotype microglial marker (iNOS) was significantly increased in LPS/IFN-γ-treated microglia, and PARP14 knockdown enhanced this effect (Figure 6D–F). CD16 and iNOS expression levels remained similar in response to various stimuli, as detected by western blot analysis (Figure 6F). In addition, LPS/IFN-γ triggered the secretion of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), whose levels increased further in response to PARP14 silencing (Figure 6G).
Figure 7|PARP14 overexpression promotes microglia M2 polarization in vitro.
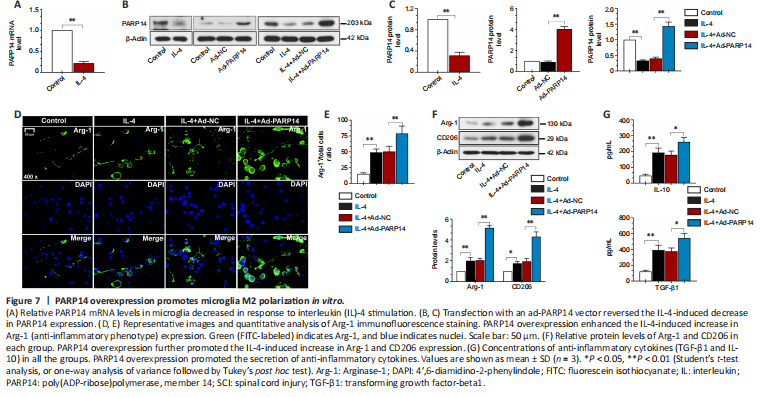
Next, we treated microglia with IL-4 to induce M2 polarization. As presented in Figure 7A–C, in response to treatment with IL-4, PARP14 mRNA and protein expression decreased markedly. To induce PARP14 overexpression, microglia were transfected with an adenovirus PARP14 overexpression vector (Ad-PARP14) or its NC (Ad-NC), and the transfection efficiency was verified by western blot analysis (Figure 7B and C). Ad-PARP14 infection significantly increased PARP14 expression in microglia stimulated with IL-4 (Figure 7B and C). The immunofluorescence staining results showed that IL-4 treatment increased Arg-1 expression, and Ad-PARP14 infection further enhanced Arg-1 expression (Figure 7D and E). Western blot analysis showed that M2 phenotype microglial marker (Arg-1 and CD206) protein levels were significantly increased in microglia stimulated with IL-4 and further elevated by PARP14 overexpression (Figure 7F). In addition, treatment with IL-4 increased the accumulation of anti-inflammatory cytokines (TGF-β1 and IL-10), and the levels of these cytokines were further increased by PARP14 overexpression (Figure 7G).
Figure 8|PARP14 regulates microglia M1/M2 polarization through the STAT1/6 pathway in vitro.

Next, we investigated the mechanism by which PARP14 regulates the microglia-mediated inflammatory response. As shown in Figure 8A and C, p-STAT1 expression was significantly increased by stimulation with LPS/IFN-γ, and PARP14 knockdown further elevated p-STAT1 expression levels. In addition, treatment with IL-4 significantly increased p-STAT6 expression, and PARP14 overexpression further enhanced this effect (Figure 8B and C).
Figure 9|PARP14 deficiency exacerbates SCI-induced bone loss (28 days post-SCI).

Rapid bone loss and osteoporosis are closely associated with SCI (Edwards et al., 2018). Therefore, we explored the effects of PARP14 on SCI-induced bone loss 28 days post-SCI. The micro-computed tomography results showed that PARP14 knockdown further decreased trabecular bone volume, trabecular thickness, and trabecular number, and increased trabecular spacing (P > 0.05; Figure 9A). Hematoxylin and eosin staining indicated that PARP14 knockdown increased SCI-induced inflammatory infiltration and the formation osteocyte lacunae, as well as exacerbating disruption of the bone structure (P > 0.05; Figure 9B). The number of osteoclasts identified histochemically by TRAP staining was increased in SCI mice and further increased by PARP14 silencing (P > 0.05; Figure 9C). In addition, PARP14 knockdown increased receptor activator of nuclear factor-kappa B ligand (RANKL) (osteoclast differentiation marker) mRNA levels but decreased osteoprotegerin (OPG) (osteogenic differentiation marker) mRNA levels (P > 0.05; Figure 9D).