脊髓损伤
-
Figure 1|Dynamic changes in gHSF1 expression in the injured gecko spinal cord.

HSF1 has been shown to protect cells against various stresses (Li et al., 2017). To elucidate the potential roles of gHSF1 in the injured spinal cord in a gecko model, 0.5-cm cord segments proximal to the amputation plane were collected 0, 1, 3, 7, and 14 days after tail amputation. Western blot analysis demonstrated that SCI significantly increased gHSF1 levels from day 1 onwards, with a peak at 3 days that returned to the control level at 14 days (P = 0.0013; Figure 1A and B). To understand how gHSF1 mediates cellular events within neurons and microglia, immunostaining was performed to observe its colocalization with markers of the two cell types following tail amputation. The results showed that gHSF1 colocalized with NeuN-positive neurons (Figure 1C) and OX42-positive microglia (Figure 1D), but not GFAP-positive astrocytes (Additional Figure 2A), before and after SCI. Notably, gHSF1 primarily localized to the nuclei, as determined by co-staining with Hoechst 33342 (Additional Figure 2B). These findings indicate that gecko SCI results in a significant increase in gHSF1 expression within neurons and microglia, and that this phenomenon is involved in successful nerve regeneration.
Figure 2|Effects of gHSF1 overexpression on gecko neuron neurite growth.
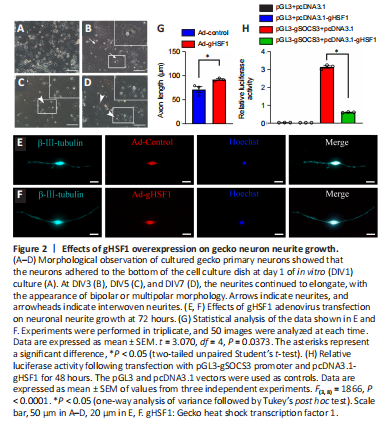
To determine the effects of gHSF1 on gecko neurons after SCI, primary neurons were isolated from the cortex and cultured as previously described (He et al., 2021b). When the primary neurons were cultured for 1 day in vitro (DIV1), most of the cells adhered to the bottom of the cell culture dish. At DIV3, however, sprouting neurites were visible on some cells (Figure 2A–D). To test the effect of gHSF1 overexpression on nerve regeneration, neurons over 95% pure, as evaluated by β-III-tubulin staining (Additional Figure 3A and B). Immunostaining showed that gecko neurons constitutively expressed gHSF1 (Additional Figure 3C). The neurons at DIV5 were transfected with gHSF1 adenovirus (Ad-gHSF1) for 72 hours, and structural analysis showed that this overexpression facilitated axonal elongation in comparison with the control cells (Figure 2E–G).
To explore how gHSF1 mediates neuronal growth, we looked for HSF1-binding elements in the promoters of several genes encoding axonal growth–related inhibitory molecules. As expected, the promoter of SOCS3, which is a critical axonal growth inhibitor (Sun et al., 2011; Gallaher and Steward, 2018), contained 12 putative HSF1-binding elements, as predicted by Jaspar analysis (https://jaspar.genereg.net/) (Additional Figure 3D and E). Therefore, the gSOCS3 promoter (–2074 to –1) was cloned into a dual-luciferase reporter gene construct. The luciferase reporter gene assay revealed that gHSF1 markedly decreased gSOCS3 expression (Figure 2H). Taken together, these findings indicate that gHSF1 promotes axonal elongation in geckos by binding to the gSOCS3 promoter and inhibiting its expression.
Figure 3|Effects of gHSF1 on neuronal apoptosis.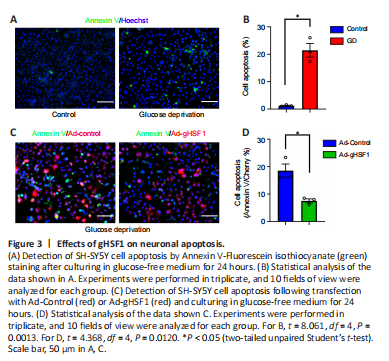
Neuronal apoptosis occurs after SCI, and HSF1 can protect neurons from death caused by the accumulation of misfolded proteins (Verma et al., 2014; Hassannejad et al., 2018). To further elucidate the pathophysiological roles of increased gHSF1 expression in gecko neurons following SCI, glucose deprivation (GD) was used to induce neuronal apoptosis (Ferretti et al., 2016). SH-SY5Y cells were cultured in glucose-free medium for 3–24 hours, which led to a marked decrease in cell viability, as determined by CCK8 assay (Additional Figure 4). Annexin V-FITC staining showed that GD for 24 hours increased the number of apoptotic cells (Figure 3A and B). However, when the cells were transfected with Ad-gHSF1 for 48 hours and then subjected to GD for 24 hours, the number of apoptotic cells was markedly reduced (Figure 3C and D). These findings indicate that gHSF1 protects neurons from apoptosis.