脊髓损伤
-
Figure 2|Bexarotene promotes functional recovery after spinal cord injury.

We established an SCI model in mice and examined the effects of Bex treatment on the functional recovery of animals after 28 days. We first examined the proportion of the collagen deposition area in the sagittal sections of the spinal cords by HE and Masson staining. While collagen deposition was significantly greater after SCI compared with sham operation, Bex treatment significantly reduced the proportion of the collagen deposition area (P < 0.01; Figure 2A and B). Immunofluorescence staining showed that MAP2 expression and the number of SYN-contacting ventral motor nerve cells were significantly greater in the SCI + Bex group compared with the SCI group after operation (P < 0.01; Figure 2C–E). Footprint analysis showed that while the stride length in the SCI group was significantly shorter than that in the sham group, the stride length in the SCI + Bex group was significantly increased compared with that of the SCI group (P < 0.01; Figure 2F and G). Analysis of BMS scores and survival curves revealed that mice in the SCI + Bex group recovered better lower limb motor function and had a lower death rate than those in the SCI group (Figure 2H and I).
Figure 3|Bexarotene attenuates neuronal pyroptosis in the spinal cord after SCI.
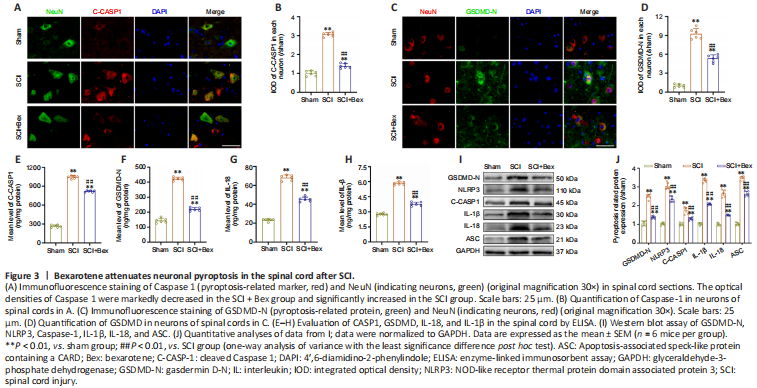
We examined the effects of Bex treatment on pyroptosis on the 5th day after SCI. High levels of pyroptosis are observed in the spinal cord after SCI and are thought to suppress motor function recovery (Al Mamun et al., 2021). Therefore, we next explored whether Bex improved functional recovery in the spinal cord after SCI by attenuating pyroptosis. We examined the expressions of GSDMD-N and Caspase-1, two critical proteins involved in pyroptosis (Shi et al., 2017), by immunofluorescence staining (Figure 3A–D). The levels of GSDMD-N and cleaved Caspase-1 were significantly increased in the SCI group compared with the sham group; furthermore, the levels were markedly decreased in the SCI + Bex group compared with the SCI group (all P < 0.01, vs. sham group; Figure 3B and D). We next examined the levels of cleaved Caspase-1, GSDMD-N, IL-1β, and IL-18 (pyroptosis markers (Al Mamun et al., 2021)) in the spinal cord by ELISA. All levels were significantly suppressed after Bex administration (P < 0.01, vs. SCI group; Figure 3E–H), suggesting that pyroptosis was reduced. Western blot results for GSDMD-N, NLRP3, Caspase-1, IL-1β, IL-18, and ASC expression levels were consistent with the immunofluorescence staining and ELISA results (Figure 3I and J). The SCI + Bex group had markedly reduced levels of these markers compared with the SCI group. Together, these results indicated that Bex suppresses pyroptosis in the spinal cord after SCI.
Figure 4|Bexarotene enhances autophagy in neurons following spinal cord injury.

We next examined the effects of Bex treatment on autophagy on the 5th day after SCI. We evaluated autophagy-related proteins and genes (p62, VPS34, Beclin1, CTSD, and LC3) in the spinal cord after SCI (Figure 4A–D). Immunofluorescence showed that the SCI group exhibited a greater proportion of LC3-positive cells than the sham group; after Bex treatment, the proportion of cells containing LC3-labelled autophagosomes was significantly greater than that in the SCI group or sham group (both P < 0.01). The levels of p62 in the SCI groups were significantly higher than that in the sham group (both P < 0.01); the level of p62 in the SCI + Bex group was markedly decreased compared with that in the SCI group (P < 0.01). Western blot analysis of spinal cord samples indicated that VPS34, p62, Beclin1, CTSD, and LC3B levels were significantly higher in the SCI group compared with the sham group; the levels of VPS34, Beclin1, CTSD, and LC3B levels were markedly increased and p62 was decreased in the SCI + Bex group compared with the SCI group (all P < 0.01; Figure 4E and F). The qPCR results indicated that the expressions of autophagy-related genes (VPS34, SQSTM1, Beclin1, CTSD and LC3) were higher in the SCI group compared with the sham group; the levels were higher in the SCI + Bex group than the SCI group (all P < 0.01; Figure 4G). These results demonstrate that autophagy was increased in the spinal cord after mild injury and Bex further upregulated autophagy in the SCI model.
Figure 5|Suppression of autophagy reverses the effect of Bexarotene on pyroptosis after SCI.
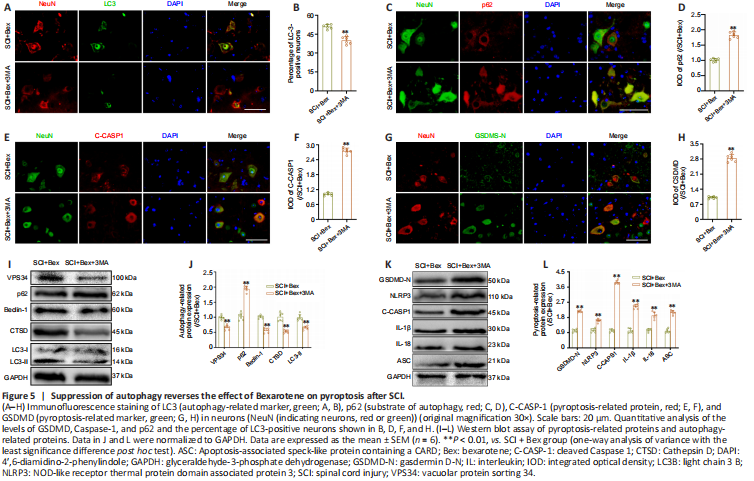
We next investigated whether Bex suppresses pyroptosis after SCI through enhancing autophagy. We administered 3MA, an autophagy inhibitor (Yang et al., 2018), to SCI model mice treated with Bex. We confirmed that the effect of Bex on enhancing autophagy was suppressed upon 3MA treatment, as shown by immunofluorescence staining for LC3 and p62 and western blot analysis of VPS34, p62, Beclin1, CTSD, and LC3II (Figure 5A–D). The SCI + Bex + 3MA group showed a significant decrease in the proportion of LC3II-positive cells and an increase in the level of p62 compared with the SCI + Bex group, as determined by immunofluorescence staining (all P < 0.01; Figure 5A–D). We next measured pyroptosis markers in the SCI + Bex and SCI + Bex + 3MA groups. Immunofluorescence staining showed that the immunopositivities of GSDMD-N and Caspase-1 were markedly increased in the SCI + Bex + 3MA group compared with levels in the SCI + Bex group (P < 0.01; Figure 5E–H). In western blot analysis: the SCI + Bex + 3MA group showed the same trend in terms of the levels for VPS34, p62, Beclin1, CTSD, and LC3II compared with levels in the SCI + Bex group (P < 0.01; Figure 5I and J). The SCI + Bex + 3MA group showed increased protein expression levels for GSDMD-N, NLRP3, Caspase-1, IL-1β, IL-18 and ASC compared with levels in the SCI + Bex group (P < 0.01; Figure 5K and L). These results indicate that 3MA inhibited the effect of Bex on reducing pyroptosis.
Figure 6|Bexarotene promotes mitophagy and decreases ROS levels after SCI.
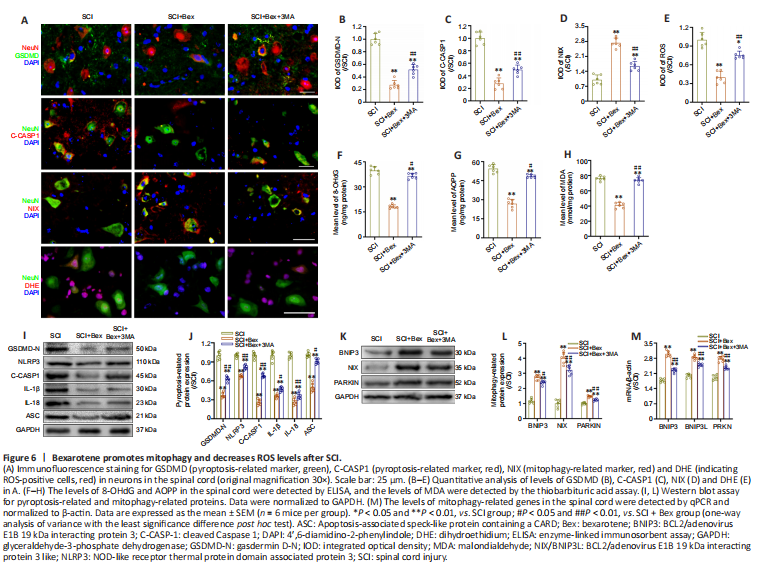
We next explored other mechanisms by which Bex suppresses pyroptosis after SCI. After SCI, ROS are upregulated in the injury area; these proinflammatory factors mediate inflammatory mechanisms, such as pyroptosis-mediated cell death (David and Kroner, 2011; Shi et al., 2015). We next measured the levels of ROS by immunofluorescence staining and ELISA. The results showed that Bex significantly attenuated ROS accumulation after SCI, along with pyroptosis, which was reversed by 3MA (all P < 0.01, Figure 6A–D; P < 0.01 in SCI group vs. SCI + Bex group, P < 0.05 in SCI + Bex group vs. SCI + Bex + 3MA group, Figure 6E). ELISA measurement of the levels of 8-OHdG, AOPP, and MDA also indicated that ROS levels were markedly decreased in the SCI + Bex group compared with the SCI group (Figure 6F–H). Western blot assay further confirmed that Bex effectively ameliorates oxidative stress after SCI (Figure 6I and J).
We next examined the effect of Bex on mitophagy by the evaluation of mitophagy-related protein levels. As shown in Figure 6K–L, the levels of BNIP3, BNIP3 L, and Parkin (markers of mitophagy) were significantly higher in the SCI + Bex group than those in the SCI group, while they were significantly lower in the SCI + Bex + 3MA group compared with the SCI + Bex group. Significantly higher levels of pyroptosis-related markers (GSDMD-N, NLRP3, Caspase-1, IL-1β, IL-18 and ASC) were observed in the SCI and SCI + Bex + 3MA groups compared with the SCI + Bex group (Figure 6I). qPCR results also demonstrated that mitophagy-related gene levels were significantly increased in the SCI + Bex group compared with the SCI group and SCI + Bex + 3MA group (P < 0.01; Figure 6M). These results indicate that Bex may reduce the levels of ROS by activating mitophagy.
Figure 7|Bexarotene promotes functional recovery following SCI via enhanced autophagy.
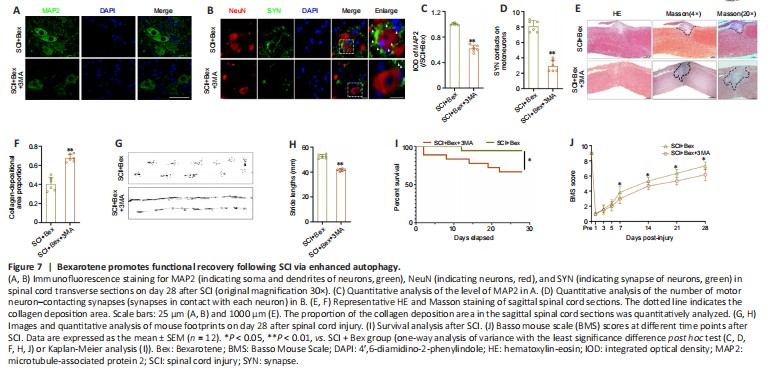
We further explored the effect of Bex on autophagy in the functional recovery of the spinal cord after SCI. The levels of MAP2 and number of SYN-positive synapses on ventral motor nerve cells were lower in the SCI + Bex + 3MA group compared with those in the SCI + Bex group (both P < 0.01; Figure 7A–D). The proportion of the collagen deposition area in the impaired spinal cord was markedly increased in the SCI + Bex + 3MA group compared with that in the SCI + Bex group (P < 0.01; Figure 7E and F). Footprint analysis showed a significantly shorter stride length in the SCI + Bex + 3MA group than that in the SCI + Bex group (P < 0.01; Figure 7G and H). The SCI + Bex group showed higher BMS scores and survival curves compared with those in the SCI + Bex + 3MA group (Figure 7I and J). These results demonstrated that autophagy and mitophagy play vital roles in SCI and that Bex improved functional recovery after SCI by influencing autophagy and mitophagy.
Figure 8|Bexarotene promotes the expression and nuclear translocation of TFE3 following SCI.
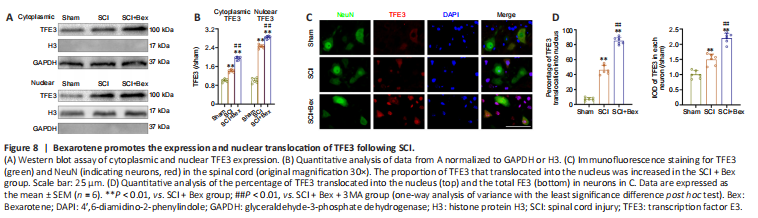
We next examined the mechanism by which Bex facilitates autophagy and mitophagy. We previously found that the TFE3 transcription factor promotes autophagy and mitophagy (Zhou et al., 2020; Lou et al., 2022). To determine whether Bex impacts autophagy and mitophagy in SCI through TFE3, we first examined TFE3 expression. Immunofluorescence staining and western blot showed that TFE3 was increased after SCI, and the increase was significantly increased in the SCI + Bex group (both P < 0.01; Figure 8). Additionally, the proportion of TFE3 that translocated into the nucleus was significantly increased in the SCI + Bex group compared with the sham and SCI groups (P < 0.01; Figure 8A–D).
Figure 9|Bexarotene inhibits pyroptosis and reduces the levels of ROS by enhancing autophagy and mitophagy by upregulating TFE3 in SCI.

To further explore the role of TFE3 in autophagy and mitophagy induced by Bex, we used TFE3 shRNA to silence TFE3 expression. Immunofluorescence results showed that the percentage of LC3II-positive cells was markedly reduced in the Bex + TFE3 shRNA group compared with the SCI + Bex + scrambled shRNA group; there was no significant difference between the SCI + Bex and SCI + Bex + scrambled shRNA group. The level of p62 was significantly higher in the Bex + TFE3 shRNA group compared with the SCI + Bex + scrambled shRNA group; no significant difference was observed between the SCI + Bex group and SCI + Bex + scrambled shRNA group (both P < 0.01; Figure 9A–J). Western blot assay revealed decreases in VPS34, Beclin-1, CTSD, and LC3II and an increase in p62 in the SCI + Bex + TFE3 shRNA group compared with the SCI + Bex group and SCI + Bex + scrambled shRNA group; no significant differences were observed between the SCI + Bex group and SCI + Bex + scrambled shRNA group (Figure 9K–L). Immunofluorescence staining for CASP-1 and GSDMD indicated that these pyroptosis-related markers, which were expressed at relatively lower levels in the SCI + Bex and SCI + Bex + scrambled shRNA groups compared with the SCI group, were significantly increased in the SCI + Bex + TFE3 shRNA group; there was no significant difference between SCI + Bex and SCI + Bex + scrambled shRNA groups. The levels of GSDMD-N, NLRP3, Caspase-1, IL-1β, IL-18, and ASC were comparable in the three groups; levels in the SCI + Bex + TFE3 shRNA group were higher than those in the SCI + Bex group and SCI + Bex + scrambled shRNA groups (P < 0.01; Figure 9M and N). Immunofluorescence staining and western blotting of mitophagy-related proteins were consistent with the change in autophagy-related markers. All levels were significantly decreased after treatment with TFE3 shRNA compared with levels in SCI + Bex and SCI + Bex + scrambled shRNA groups (all P < 0.01, Figure 9A, G, O and P).
ROS was detected by immunofluorescent staining with DHE staining. The results showed that ROS accumulation was significantly increased in the SCI + Bex + TFE3 shRNA group compared with the SCI + Bex + scrambled shRNA group; no significant differences were observed between the SCI + Bex group and the SCI + Bex + scrambled shRNA group (all P < 0.01, vs. SCI + Bex and SCI + Bex + scrambled shRNA groups; Figure 9A). ELISA for 8-OHdG, AOPP, and MDA levels showed the same trend as the immunofluorescent staining results (all P < 0.01; Figure 9Q–S). These results indicated that TFE3 is required for Bex facilitation of mitophagy-mediated reduction of ROS accumulation.