脊髓损伤
-
Figure 2|The use of magnetic nanoparticles-loaded EVs for organ targeting.
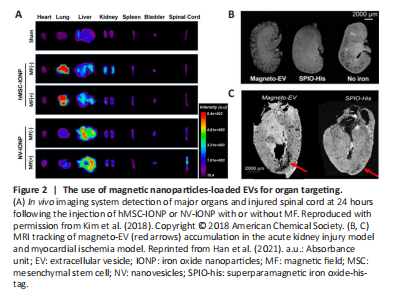
The insufficient therapeutic efficacy of EVs following systemic injection could be a result of their poor organ-targeting ability (Wiklander et al., 2015). Therefore, preventing off-target consequences is a key prerequisite for applying EV-based therapies in the repair of SCI. To this end, and inspired by the magnetic navigation theory, Kim et al. (2018) cultured iron oxide nanoparticle (IONP)-treated MSCs and then extracted INOP-incorporated exosome-mimetic nanovesicles (NV-IONP). The therapeutic effect of NV-IONP was then evaluated in a model of spinal cord compression. Interestingly, IONP priming encouraged the release of larger amounts of pro-regenerative growth factors inside EVs by activating the c-Jun N-terminal kinase and c-Jun signaling pathways. The intravenous injection of NV-IONP led to approximately four-fold higher IONP accumulation in the lesion than MSC-IONP. Furthermore, when an external magnetic field was applied, the NV-IONP produced a two-fold increase in targeting efficiency when compared to NV-IONP delivered without magnetic navigation. With enhanced organ-targeting efficiency under magnetic guidance, NV-IONP induced remarkable blood vessel formation, attenuated apoptosis and inflammation, and consequently, restored lost motor function (Figure 2A; Kim et al., 2018). While satisfactory outcomes were achieved, the in vivo tracking method of NV-IONP should be improved. Lipophilic fluorescence labeling technology was adopted to track the in vivo biodistribution of NV-IONP, but this could not fully represent the true fate of NV-IONP, predominantly due to the aggregation of lipophilic dye or restricted tissue penetration depth under fluorescence imaging (Betzer et al., 2020). In this sense, combining other tracking modalities, such as high-resolution magnetic resonance imaging scanning, could provide more convincing evidence for the accumulation of NV-IONP in an SCI lesion, as this technology has been previously demonstrated to permit the in vivo tracking of therapeutic EVs in different animal models of myocardial ischemia and kidney injury (Figure 2B and C; Han et al., 2021).
Figure 3|Representative combinations of biomaterials and EVs for the treatment of SCI.
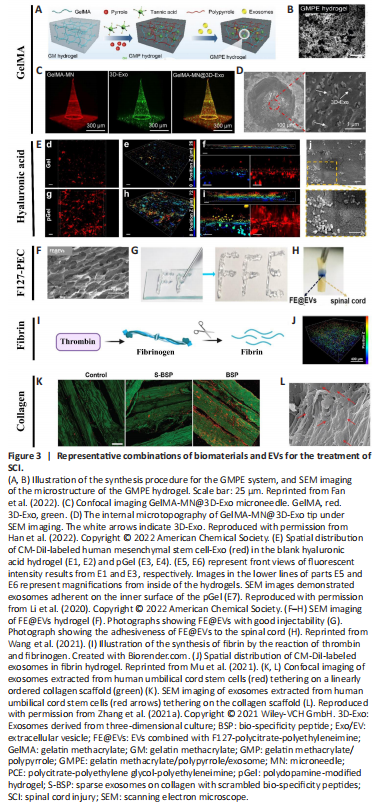
Although the combination of biomaterials and stem cells has been widely used and tested in preclinical SCI models, the use of biomaterials in conjunction with EVs is relatively less common. Yet, as one of the most emerging and promising “cell-free” tools in the field of regenerative medicine, EVs are increasingly being used with biomaterials, and recently such combinations have been reported as pro-regenerative in rodents with SCI. Thus, it was important that we review recent advances in the use of biomaterials with EVs for SCI repair (Figure 3).
Gelatin methacrylate (GelMA) has been widely used to treat nerve injuries, predominantly due to its favorable biocompatibility and biodegradability. For instance, GelMA hydrogel has been used to promote 3D neuronal differentiation of bone marrow-derived mesenchymal stem cells (BMSCs) and NSCs for spinal cord regeneration (Zhou et al., 2020). Recently, combining EVs with various forms of GelMA hydrogel has emerged as an attractive approach for repairing SCI.
Since the soft and hydrated forms of hydrogels resemble native nerve tissues, hydrogels are extensively used to promote tissue formation after SCI. However, one of the major obstacles in translating hydrogel-based therapies to regulate excitable cells such as nerve cells is their poor conductivity (Wu et al., 2016). In this regard, electroconductive hydrogels are extremely attractive in terms of mimicking the electrical transmission properties of native nerve tissues. Such hydrogels are composed of a hydrophilic matrix and conduct substances such as carbon materials, metallic nanoparticles, and electroconductive polymers (Wang et al., 2010). Despite having both mechanical and electrical properties, electroconductive hydrogels could potentially trigger immune responses to foreign bodies (Liu et al., 2021). To mitigate this detrimental effect while utilizing the advantages of electroconductive hydrogels, Fan et al. (2022) loaded electroconductive hydrogels made of GelMA, polypyrrole (PPy), and tannic acid (TA), with BMSC-derived EVs that exhibited immunomodulatory effects for the repair of damaged tissues or organs. Transplantation of the GMPE system (GelMA-PPy-TA-Exosomes) into SCI lesions effectively modulated microglial polarization, recruited endogenous NSCs and further differentiated them into neurons and oligodendrocytes; most importantly, they promoted significant functional recovery in a model of severe SCI (Figure 3A and B; Fan et al., 2022).
Currently, the most prevalent method used for EV-biomaterial therapy is local implantation/injection into the SCI site. While this method avoids potential systemic effects and poor bioavailability as would occur following intravenous delivery, this practice could cause additional damage to the spinal cord tissue. Subsequently, an innovative approach was developed which utilized a GelMA-based microneedle array patch to store 200 μg MSC-EVs obtained from 3D culture (GelMA-MN@3D-Exo) for implantation at the SCI site. GelMA-MN@3D-Exo patches significantly enhanced the 3D-Exo retention rate, achieved controlled release, reduced neuroinflammation, and improved nerve function recovery (Figure 3C and D; Han et al., 2022).