脊髓损伤
-
Figure 1|Graft survival 5 days after transplantation into the spinal cord of SCI rats.
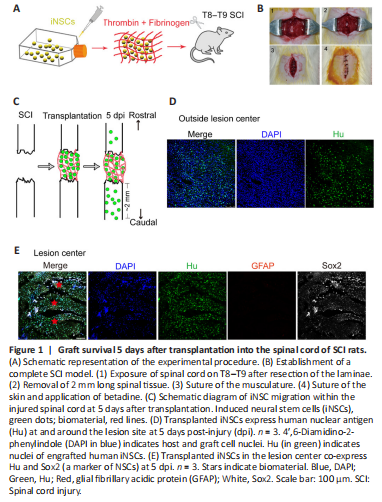
In our previous study, we successfully converted human PBMCs into iNSCs (Yuan et al., 2018). Importantly, in vivo transplantation of either iNSCs or iNSC-derived dopaminergic precursors into the brain of immunodeficient mice did not cause tumor formation (Yuan et al., 2018). Here, iNSCs were expanded in proliferation medium for several passages, dissociated into single cells, and then mixed with fibrinogen and thrombin to form a soft clot. This clot was then transplanted into the lesion center of the completely transected spinal cord (Figure 1A and B and Additional Figure 3). Once mixed, the material and cells solidify rapidly (usually within 3 seconds) at room temperature. Five days post-injury, Hu+ cells (stained for human nuclei, a marker of human cells) were detected at the lesion site (Figure 1C and E). Some grafted cells (36% of total grafted cells) were also observed beyond the lesion site, mostly in areas caudal to the lesion boundary, at a distance up to 2 mm (Figure 1C and D). In addition, Hu+ cells were co-labeled with the NSC marker, Sox2, but were negative for GFAP, a marker for astrocytes that usually stains negative at the lesion core and can be used to define the lesion boundary (Figure 1E). However, Hu+ cells failed to be detected by day 15 onward after injury (data not shown). These results suggest that the transplanted iNSCs could survive at least short-term in the injured spinal cord.
Figure 3|SCI+iNSCs transplantation group shows reduced lesion volume compared with SCI only group at 7 months post-injury.
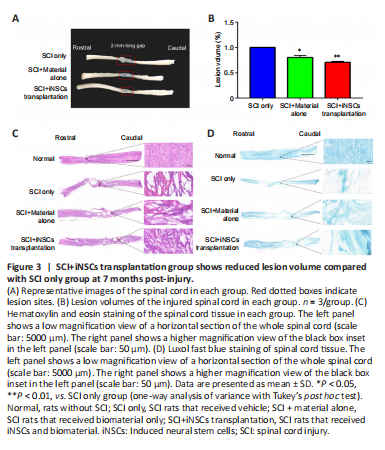
The average lesion length was 4.1, 3.2, and 2.5 mm in the SCI only, SCI + material alone, and SCI + iNSCs transplantation groups, respectively (Figure 3A). Activated astrocytes (which are GFAP-positive) can be used to mark the border of spinal cord lesion areas. Using GFAP staining, the lesion volume in each group was calculated. Engraftment of iNSCs with material markedly reduced the lesion volume, which might have contributed to the functional recovery (Figure 3B). In addition, H&E staining was performed to examine tissue morphology (Figure 3C). Following SCI, the spinal cord exhibited a porous morphology with reduced cellular density (Figure 3C). This was reversed by transplantation with iNSCs-material (Figure 3C). Similarly, LFB staining to examine myelination, indicated a larger area of myelination at the lesion site in the iNSCs-material group compared with SCI only group (Figure 3D). These results suggest that engraftment of iNSCs with material can reduce the lesion volume and improve axon myelination, which might account for the functional recovery.
Figure 4|Degradation of fibrin, Tuj1, and NF200 expression at the lesion core and around the lesion border in each group 7 months after SCI.
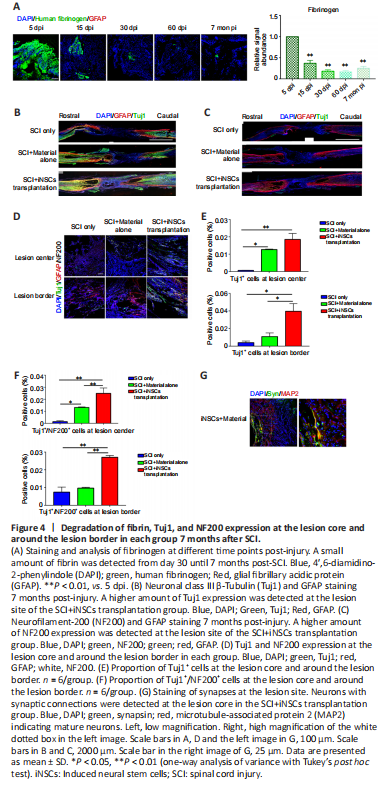
Transplanted iNSCs showed good survival on day 5 but this was gradually diminished over time. From day 15 onward, no transplanted iNSCs were detected in the spinal cord. To investigate what might account for the long-term functional benefits at 7 months post-transplantation, we examined the kinetics of the biomaterial used in the current study. Fibrinogen-specific antibody detects fibrin and can be used to examine kinetics of the biomaterial (Figure 4A and Additional Figure 4). Specifically, at 5 days post-injury/transplantation, a large amount of fibrin remained at the engraftment site (Figure 4A and Additional Figure 3). However, the biomaterial gradually degraded, with a significant decrease in quantity at 15 dpi. From 30 dpi onward, only a small amount of material was occasionally detected (Figure 4A). We also examined the presence of neuronal axons at the lesion core. Using GFAP staining to define the lesion borders, we stained spinal cord tissue sections for Tuj1 (a marker for immature neurons at the early stage of neuronal differentiation) and for NF200 (a marker for mature neurons). Compared with the SCI only group, some Tuj1 signal (Figure 4B) and NF200 signal (Figure 4C) (0.02–0.03%) was observed at the core of the lesion at lower magnification. At higher magnification (Figure 4D), only a few Tuj1+ or Tuj1+/NF200+ cells were detected at the lesion core in the SCI only group. At the core and border of the lesion center, a greater abundance of Tuj1+ and Tuj1+/NF200+ signals were detected in the iNSCs-material group (Figure 4D–F). The material alone group also showed a greater abundance of Tuj1+ and Tuj1+/NF200+ signals at the lesion core, in comparison with the SCI only group. Furthermore, by staining for MAP2 (a marker of mature neurons), and synapsin (a marker of presynaptic terminals), we detected synapsin+/MAP2+ possibly innervated axons at the lesion core in the iNSCs-material group, but not in the SCI only group (Figure 4G). These results indicate that engraftment of iNSCs and biomaterial could have led to the generation of functional axons at the lesion site.
Figure 5|BDNF, TGFβ, and TNFα expression in control, material, and SCI+iNSCs transplantation groups.
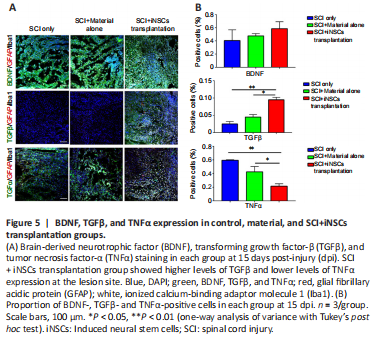
Figure 6|SCI + iNSCs transplantation group shows reduced inflammation response after SCI.
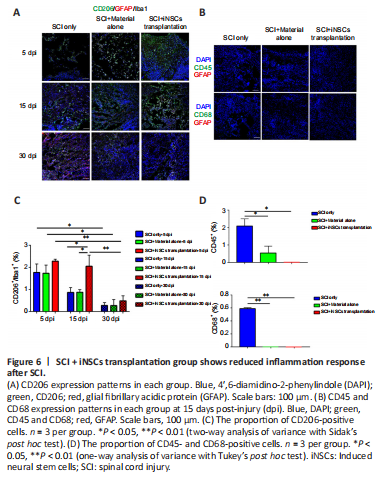
Figure 7|Engraftment of iNSCs with material does not change the type of scar tissue 7 months post-injury.
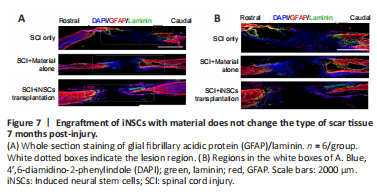
Transplanted iNSCs showed good survival at 5 dpi, but this was gradually diminished over time. No transplanted iNSCs were detected from day 15 onward. Nevertheless, the effect on motor function and pathology appears to be long-lasting. One possibility was that during the acute and/or subacute stages following SCI, the hostile microenvironment might have abolished the key step(s) involved in endogenous axonal regeneration and/or neurogenesis. Modulation of the niche at the early stage might have a long-lasting effect on pathology and function. To address this question, we examined niche components of spinal cord tissue sections at 15 dpi. Staining for the trophic factors, brain-derived neurotrophic factor (BDNF), insulin-like growth factor 1, and neurotrophin-3, showed that only BDNF was detected, but with no significant difference observed between the three groups (Figure 5A and B). Staining for the cytokines, IL4, IL6, tumor necrosis factor-α (TNFα), and transforming growth factor-β (TGFβ) revealed that TGFβ expression was increased while TNFα expression was reduced in the iNSCs-material group (Figure 5A and B). Neither IL4+ nor IL6+ cells were detected at the lesion site at this time point. In addition, staining for the corresponding receptors, TrkB (receptor of BDNF), TGFβR1 (receptor of TGFβ), and TNFR1 (receptor of TNFα) showed lower expression of TGFβR1 and TrkB in the lesion core after SCI (Additional Figure 5A). TGFβR1 expression was elevated and TNFR1 markedly reduced after transplantation of iNSCs (Additional Figure 5B). No statistical significance was detected for TrkB staining among the three groups. These results are consistent with the previous staining results (Figure 5). Further results showed that the number of CD206+/Iba1+ cells (M2 microglia) was greater in the iNSCs-material group on 15 dpi, compared with the SCI only and SCI+material alone groups (Figure 6A and C). Staining of CD86 (a marker of M1 macrophages) showed that CD86+ cells were reduced at 15 dpi and 30 dpi (Additional Figure 6A). On 5 dpi and 15 dpi, the quantity of CD86+ cells decreased following fibrin and/or iNSCs transplantation (Additional Figure 6B). On 30 dpi, there was no significant difference among the three groups (Additional Figure 6B). We believe that this might be due to the suppression of inflammatory responses and transition of macrophages/microglia to a M0 state at 30 dpi. We also examined immune cells by staining for CD45 and CD68, finding a reduced number of both CD45+ and CD68+ cells in the iNSCs-material group (Figure 6B and D). Previous studies have shown that the extracellular molecule, laminin, stimulated the growth of neurites from dorsal root ganglion neurons (Plantman et al., 2008; Anderson et al., 2016), therefore we examined laminin expression in each group. Staining for laminin and GFAP (Figure 7) revealed that the grafts did not alter the subtypes of activated astrocytes at the lesion site. These results indicate that iNSCs improved the microenvironment post-SCI, which might be beneficial for regeneration.