脑损伤
-
Figure 1|Focal infarction is restricted to the right somatosensory cortex, which regulates left forelimb function.
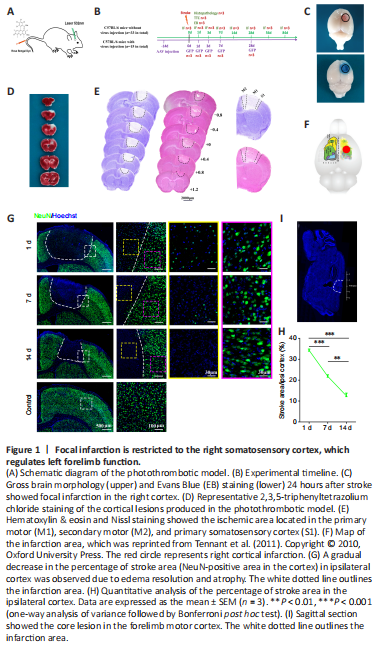
结果:To induce focal ischemic injury of the sensorimotor cortex, a photothrombotic (PT) model was established using a modified version of a previously described protocol (Joy et al., 2019). Briefly, each mouse was intraperitoneally injected with 1% pentobarbital sodium (50 mg/kg, Cat# Tc-P8411, Merck, Darmstadt, Germany), and the head was immobilized in a stereotactic apparatus (68505, RWD Lifescience, Beijing, China). A window was drilled into the skull above the forelimb area of the right motor cortex reported in a microsimulation study (Tennant et al., 2011) at the following coordinates relative to the bregma: AP: –0.5 mm to 1 mm, ML: –0.75 mm to –2.25 mm. Rose Bengal solution (10 mg/mL) was administrated by tail vein injection at a dose of 80 mL/kg. Then, the cortex visible through the skull window was illuminated for 2 minutes using a cold light source (KL1500 LCD, Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA) that delivered a 1.5-mm diameter region of illumination from 1.0 cm away from skull window (Figure 1A). In the control group, mice underwent the same surgical procedure without illumination. The animals were euthanized at 1, 3, 7, 14, 28, 56, and 84 days following ischemic injury. The experimental timeline is shown in Figure 1B.
To accurately identify the area of cortical infarction, cerebral morphology was assessed 24 hours after surgery. Gross brain morphology and EB staining showed the existence of a circular, 2-mm-diameter focal infarction predominantly located in the right cortex of PT mice (Figure 1C). TTC staining showed U-shaped lesions that extended through all layers of the cortex but did not cross the subcortical callosum (Figure 1D). H&E and Nissl staining further confirmed that the ischemic area was located approximately 1.2 mm rostral to 0.8 mm caudal to the bregma and 0.5 mm to 2.5 mm lateral in the primary motor, secondary motor, and primary somatosensory cortical areas (Figure 1E). This area overlapped well with the caudal forelimb area, which was defined in mice through microsimulation study (Tennant et al., 2011) (Figure 1F).
Next, we used the mature neuron marker NeuN (Hao et al., 2017) to characterize pathological changes in neurons following stroke. At day 1 post-injury, a well-defined infarction had formed, most necrotic neurons had disintegrated, and only a few pyknotic NeuN-positive cells were detected. Almost no neurons survived to day 7, and the ischemic cortex showed severe atrophy on day 14 post-injury (Figure G). The stroke area (NeuN- area in the cortex) occupied 34.22 ± 1.12%, 21.80 ± 1.31%, and 12.51 ± 2.20% of the ipsilateral hemisphere at days 1, 7, and 14, respectively, following stroke (day 7 vs. day 1: P < 0.001; day 14 vs. day 1: P < 0.001; day 7 vs. day 14: P < 0.01; Figure 1H). Further examination demonstrated that the area of neuronal death did not expand to the rostral border of the hindlimb motor cortex (did not extend further caudal than 1.0 mm posterior to the bregma) (Figure 1I). Taken together, these results suggest that ischemic death of neural somas was restricted to the defined forelimb representation of the right sensorimotor cortex in a PT mouse model of stroke.
Figure 2|An anti-APP antibody exclusively targeted axonal degeneration within the ischemic cortex.
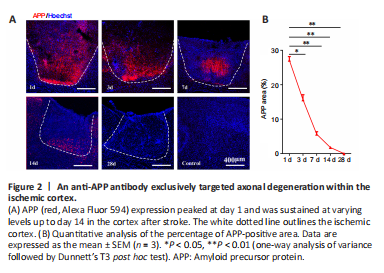
结果:CST axons from the forelimb representation of the sensorimotor cortex primarily terminate at the cervical and thoracic levels (Steward et al., 2021). Thus, we selected the right pyramid and the base of the dorsal column of the left cervicothoracic spinal cord as the ROIs for examining axonal degeneration. Since APP is transported via fast anterograde axonal transport, APP accumulation is considered to be a sensitive marker of axonal injury (Stone et al., 2000). We therefore performed anti-APP immunofluorescence staining to observe axonal degeneration in the CST following ischemic death of the neuronal somas. As shown in Figure 2, APP expression peaked at day 1 and was sustained at varying levels up to day 14 after stroke (day 3 vs. day 1: P < 0.05; days 7–28 vs. day 1: P < 0.01). Notably, APP-positive deposition was primarily observed in the ischemic cortex but was not detected in remote areas of the CST (Additional Figure 1). These results suggest that the anti-APP antibody we used exclusively detected axonal injury within the proximal disconnected segment, with virtually no evidence of distal immunoreactivity at any time point. Thus, APP may not be an ideal indicator for identifying axonal degeneration in the CST following stroke.
Figure 3|Visualization of WD utilizing an orthograde virus expressing GFP.
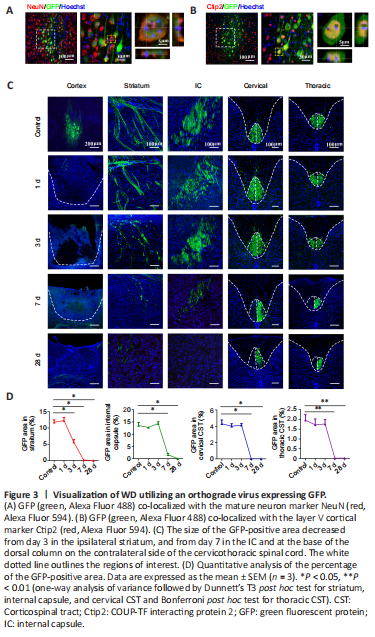
结果:To visually label axons within the CST, we injected the orthograde virus AAV2/9-hSyn-EGFP into the right forelimb representation. Fourteen days after injection of the virus, GFP co-localized with the neuron marker NeuN (Figure 3A) and the layer V cortical marker Ctip2 (Figure 3B). These observations confirmed that the orthograde virus successfully infected pyramidal neurons located in the deep layer V of the sensorimotor area and their full-length CST projections, including those located in the ipsilateral striatum, internal capsule, medullary pyramid, and base of the dorsal column of the contralateral side of the cervicothoracic spinal cord (Figure 3C).
Figure 4|Time course of proliferated astrocyte activity following stroke.
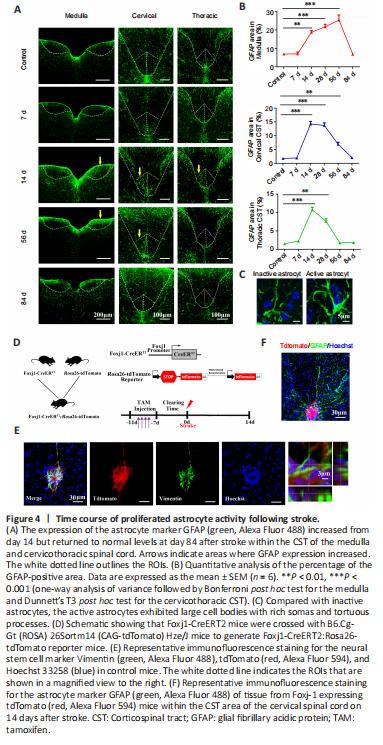
结果:Progressive destruction of fiber structures during WD causes gliosis (Nagamoto-Combs et al., 2010). Therefore, we next used GFAP immunostaining to assess the astrocyte response to WD in the CST. In the control group, a basal level of GFAP+ cells with stellate morphology was observed within the CST. On day 7 post-injury, no obvious changes were observed in the size of the GFAP+ area within the affected CST (P > 0.05, vs. control group), indicating that there was no immediate astrocyte response to WD in the acute stage of stroke. The size of the GFAP+ area increased sharply on day 14 post-injury (medulla: P < 0.01; cervical and thoracic CST: P < 0.001. vs. control group) and remained high on day 28 (medulla and cervical CST: P < 0.001; thoracic CST: P < 0.01; vs. control group; Figure 4A and B). These proliferated astrocytes exhibited large cell bodies with rich somas and tortuous processes, indicating hypertrophic morphology (Figure 4C). However, no significant difference in the size of the GFAP+ area was found on day 84 post-injury (P > 0.05, vs. control group; Figure 4A and B). These results demonstrate that astrocytes exhibited relatively delayed mobilization as well as a moderate response to injury and WD.
Endogenous NSCs in the central canal of mammalian spinal cords can be activated, proliferate, and differentiate into glial cells in response to injury (Liu and Chen, 2019). To determine whether the GFAP+ astrocytes that we observed after stroke were generated from NSCs, we used Foxj1-CreERT2:Rosa26-tdTomato reporter mice to label ependymal cells (Figure 4D). As shown in Figure 4E, all Foxj1-expressing cells appeared red after TAM induction. However, no tdTomato+ cells proliferated and migrated to the base of the dorsal column of the left cervicothoracic spinal cord on day 14 after stroke. In addition, no GFAP+ astrocytes colocalized with tdTomato+ cells in the CST region (Figure 4F). These results suggest that the activated astrocytes observed in the CST during WD did not differentiate from endogenous NSCs in the spinal cord.
Figure 5|Microglial recruitment and activation within the CST following stroke.
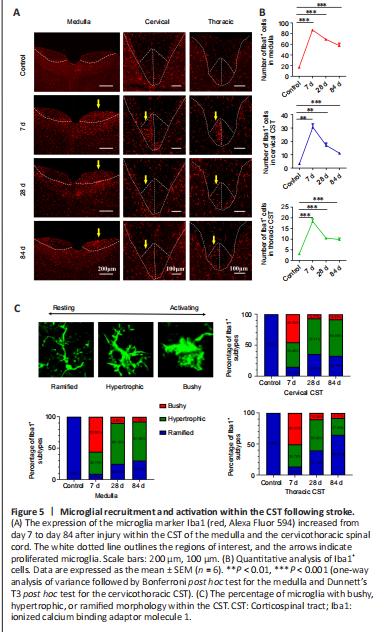
结果:To detect microglial recruitment and activation within the CST following cortical ischemic injury, we performed Iba1 immunostaining. Compared with the control group, significant increases in the number of Iba1+ microglia were observed in both the pyramidal tract and the cervicothoracic CST in PT mice (Figure 5A). These increases peaked on day 7, when most microglia showed amoeboid activation with bushy morphology (medulla and thoracic CST: P < 0.001; cervical CST: P < 0.01. vs. control group). Afterward, the number of microglia gradually declined but remained significantly high on day 84 post-ischemic injury (P < 0.001, vs. control group; Figure 5B). Notably, the microglia gradually became hypertrophic and even adopted a ramified morphology over time (Figure 5C). These results suggest that microglia were recruited but failed to maintain a persistent phagocytic state within the affected areas of the CST.
Figure 6|Wallerian degeneration of the corticospinal tract does not induce transneuronal degeneration of spinal anterior horn cells 84 days after stroke.
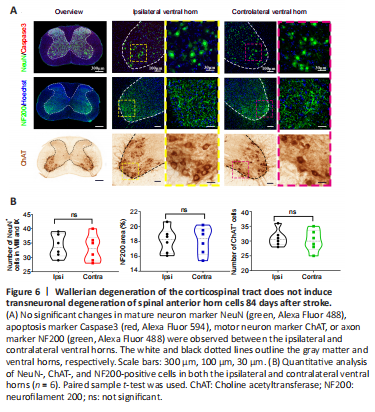
结果:To determine whether WD of the CST caused by upper motor neuron lesions in stroke further induces transneuronal degeneration of lower motor neurons, we combined NeuN labeling with immunostaining using the apoptotic marker Caspase3. As shown in Figure 6A, no NeuN+ neurons colocalized with Caspase3, indicating that no neuronal apoptosis occurred in either the dorsal or ventral horn on either the affected or the unaffected side 84 days after stroke in the cervical segment. Next, we examined the number of motor neurons in the ventral horn and found that there was no marked change in the number of NeuN+ cells in lamina VIII and IX between the ipsilateral and contralateral ventral horns (P > 0.05; Figure 6B). In addition, there were no significant spatial or quantitative changes in immunoreactivity with ChAT, a specific indicator of motor neurons, compared with the unaffected side (P > 0.05; Figure 6). Lastly, we performed NF200 immunofluorescence staining to examine axon density in the ventral horn. Compared with the contralateral ventral horn, no changes in the size of the NF200-positive fluorescence area were found in the ipsilateral ventral horn (P > 0.05; Figure 6). Collectively, these results suggest that there is no anterograde transneuronal degeneration of lower motor neurons 84 days after ischemic damage to upper motor neurons.
点击此处查看全文