脑损伤
-
Figure 2|Exercise suppresses neuroinflammation and restores PV-interneurons in the mouse model of epilepsy.
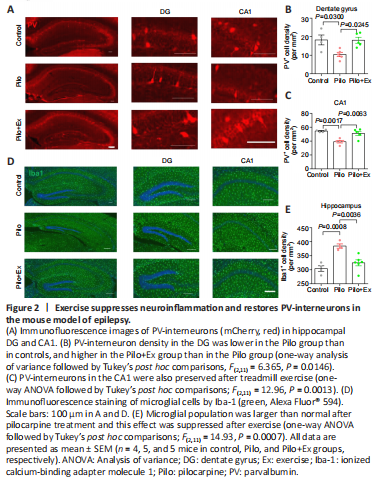
Hippocampal synaptic plasticity is under tight control of PV-interneurons (Seo et al., 2021), which have been reported to be the phagocytotic target of microglial cells (Crapser et al., 2020). Since epilepsy frequently induces neuroinflammation and microglial activation (Jiang et al., 2021), we next investigated the potential involvement of microglial cells and PV-interneurons in the exercise effect. Immunofluorescence staining showed that the density of PV-interneurons in the dentate gyrus (DG) and CA1 regions of hippocampus were markedly lower in the Pilo group than in controls. In contrast, PV-interneuron density was increased in the Pilo+Ex group (Figure 2A–C). Moreover, we found that compared with controls, the density of microglial cells was higher in the Pilo group, and the microglial population was decreased in the Pilo+Ex group (Figure 2D and E).
Figure 3|Activation of microglial cells and neuroinflammation in the mouse model of epilepsy with regular exercise.

The reactivity of microglial populations has been characterized by the presence of lysosomal protein CD68 (Bernal et al., 2002). We found that CD68 levels were elevated in Pilo group, and this was significantly alleviated in Pilo+Ex group (Figure 3A and B). We also performed a morphometric study of individual microglial cell dendrites (Figure 3C), and analysis showed decreased dendritic complexity in the model mice, as well as the improvement of cellular morphology in the model mice that were allowed to exercise (Figure 3D and E). The morphometric data and the lower levels of inflammation after exercise (indexed by levels of inflammatory cytokines: interleukin-6 and tumor necrotic factor-α; Figure 3F and G) converged to support the conclusion that neuroinflammation improved as a result of treadmill exercise.
Figure 4|Neuroinflammation leads to PV-interneuron loss and memory deficits in the mouse model of epilepsy with regular exercise.

结果:To further illustrate the role of neuroinflammation in the depopulation of PV-interneurons during epilepsy, we fed mice PLX5622, an inhibitor of colony-stimulating factor 1 receptor, to eliminate microglial cells (Walter and Crews, 2017). Two-week oral administration of PLX5622 effectively decreased the density of microglial cells in hippocampus (Figure 4A–C). As a consequence, PV-interneuron density was markedly elevated in Pilo group (Figure 4D; one-way ANOVA, P < 0.0001; Figure 4E; one-way ANOVA, P = 0.0007; Figure 4F), supporting the role of microglial cells in depleting the hippocampus of PV-interneurons. Moreover, behavioral assays showed that PLX5622 treatment improved memory performance in Pilo treated mice (one-way ANOVA, P = 0.1846; Figure 4G; one-way ANOVA, P = 0.0005; Figure 4H; one-way ANOVA, P = 0.0003; Figure 4I; one-way ANOVA, P < 0.0001; Figure 4J), highlighting the participation of neuroinflammation in the pathogenesis of epilepsy-associated memory deficits.
Figure 5|Exercise training maintained BBB integrity and relieved neuroinflammation in the mouse model of epilepsy.
结果:Lastly, to further investigate the neuroinflammation mechanism in this epilepsy model, we examined the permeability of the BBB, whose leakage amplifies the severity of neuroinflammation (Takata et al., 2021). After intravenous injection of Evans blue dye, the Pilo group of mice consistently showed higher concentrations of dye in brain tissue than did the control group (Figure 5A and B), suggesting that the BBB is compromised in this epilepsy model. Conversely, exercise improved BBB function, as evidenced the lower amounts of dye in the brain (Figure 5C). Additionally, we further assessed the structural integrity of the BBB by quantifying expression levels of Claudin-5 (Cldn5), a critical component of the vascular barrier (Yang et al., 2021). We found that pilocarpine treatment significantly decreased Claudin-5 levels, and that either regular exercise or PLX5622 increased its expression levels (Figure 5D). PLX5622 treatment also reduced the reactivity of microglial cells, as evidenced by lower CD68 fluorescent-signal intensity (Figure 5E and F). In summary, our molecular, electrophysiological, and behavioral data converged to show that hippocampal plasticity and memory function improved in a mouse model of epilepsy after 2 weeks of regular exercise.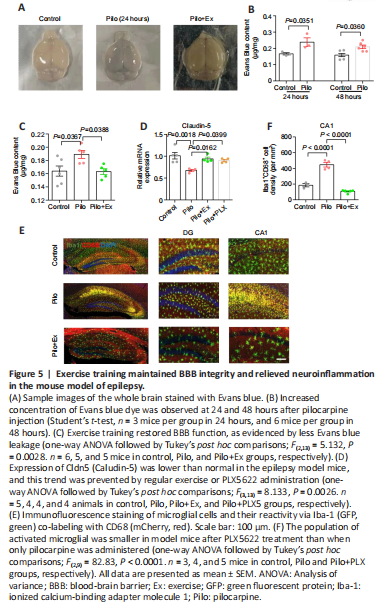
点击此处查看全文