脑损伤
-
Figure 1|Neurons but not glial cells were infected by the AAV2-GFP virus in the DG region of the hippocampus.
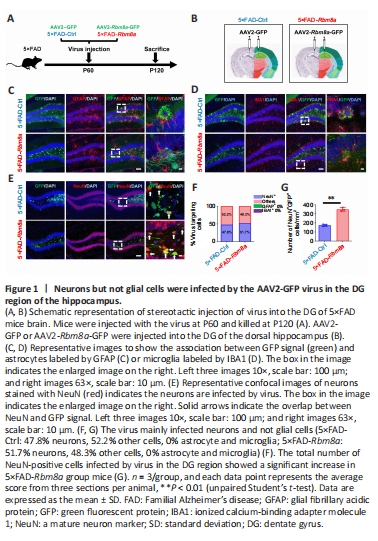
qRT-PCR and western blotting were performed to investigate age-related Rbm8a changes in 1, 2, 6, and 12-month WT mice, revealing a marked decrease in Rbm8a expression with aging (P < 0.01 or P < 0.05; Additional Figure 1A–C). Additionally, potential changes in Rbm8a expression were explored in 5×FAD mice, showing statistically significant decreases compared with WT mice (P < 0.05; Additional Figure 1D and E). Moreover, 5×FAD mice had significantly higher levels of APP compared with WT mice (P < 0.05; Additional Figure 1D and E), which confirmed the validity of the 5×FAD mice as an AD mouse model. Based on these observations, overexpression of Rbm8a might represent a viable therapeutic strategy for addressing the cognitive decline in AD. Here, we generated a Rbm8a overexpression virus and its control virus (Additional Figure 2A). Immunofluorescence staining of Rbm8a confirmed Rbm8a overexpression mediated by AAV infection, as indicated by co-immunostaining of Rbm8a (red) and AAV-GFP (green) (Additional Figure 2B). We quantified 3×FLAG-tag and RBM8A protein expression in the DG of 5×FAD-Ctrl and 5×FAD-Rbm8a mice. 5×FAD-Rbm8a mice showed significantly elevated expression of 3xFLAG-tag and RBM8A compared with the 5×FAD-Ctrl group (P < 0.01; Additional Figure 2C–E). To confirm the specific cell types targeted by AAV, an experiment was designed (Figure 1A and B) to label common cell types within the DG region (including astrocytes, microglia, and neurons) using immunofluorescence staining. The results revealed that AAV primarily infected mature neurons, constituting about 47.5% to 51.7% of infected cells (Figure 1C–F), with no observed infection in astrocytes or microglia. Additionally, other infected cells were speculated to be neural cells such as stem cells, neural progenitor cells, and immature neurons. The number of mature neurons infected by AAV in the DG was quantified, indicating a statistically significant increase in the 5×FAD-Rbm8a group compared with the control group (P < 0.01; Figure 1G). These results strongly supported the notion that Rbm8a plays an integral role in the production of neurons.
Figure 2|Reduced soluble Aβ and plaque formation in the DG of 5×FAD mice with Rbm8a overexpression.
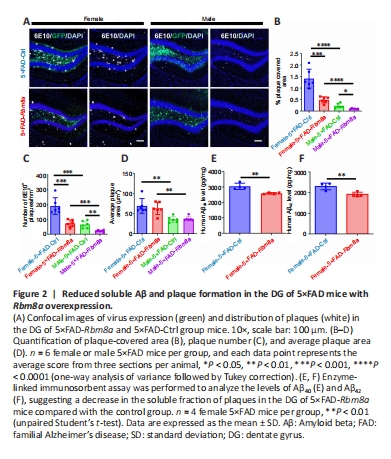
Extensive studies have shown that the accumulation of amyloid protein in the brain is a pathological hallmark of the onset and progression of AD (Abdul-Hay et al., 2012). This study’s primary goal was to determine if augmenting the expression of RBM8A can mitigate amyloid protein deposition and plaque formation in AD. Therefore, immunofluorescence staining was performed to analyze brain slices with Aβ-specific antibodies (Figure 2A), revealing a significant decrease in plaque coverage area (P < 0.001 or P < 0.05; Figure 2B) and an overall reduction in the number of plaques present (P < 0.001 or P < 0.01; Figure 2C) among both male and female 5×FAD mice following Rbm8a overexpression in the DG. Interestingly, no changes were noted in terms of individual plaque size within the 5×FAD-Rbm8a experimental group compared with the 5×FAD-Ctrl group (P > 0.05; Figure 2D). Additionally, our results revealed that females in both the 5×FAD-Rbm8a and 5×FAD-Ctrl groups had a greater plaque-covered area and number of plaques within the DG region compared with males (P < 0.01 or P < 0.001; Figure 2A–D). ELISA testing was used to analyze the levels of soluble human Aβ40 and Aβ42 within the DG region of female 5×FAD mouse brain. The findings revealed a significant reduction in soluble Aβ production in 5×FAD-Rbm8a mice compared with 5×FAD-Ctrl mice (P < 0.01; Figure 2E and F). Hence, elevating RBM8A levels could potentially be an effective approach to reduce amyloid buildup in individuals with AD.
Figure 3|Reduced dystrophic neurites surrounding plaques following overexpression of Rbm8a in the DG of 5×FAD mice.
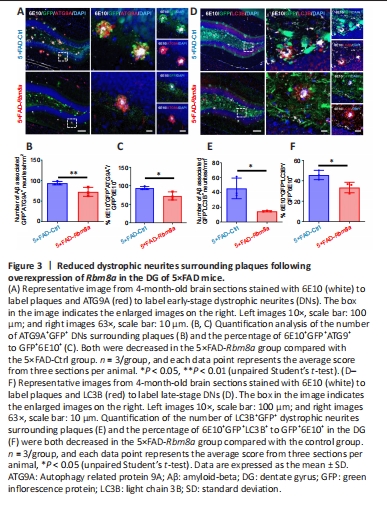
In the DG region of 5×FAD mice, a disrupted GFP signal was observed near plaques that appeared to be DNs, characterized by an misshapen cell morphology and higher GFP signal intensity. This indicates more GFP+ cells might be impaired in 5×FAD-Ctrl mice, with reduced cell loss in 5×FAD-Rbm8a mice. The disrupted GFP signal was confirmed by both anti-ATG9A (Figure 3A) and anti-LC3B (Figure 3D) markers. As opposed to ATG9A (a marker for early-onset DNs), LC3B is a marker for late-onset DNs, which are distributed relatively further from the core of amyloid plaques, as previously reported (Sharoar et al., 2019). Compared with the 5×FAD-Ctrl group, mice in the 5×FAD-Rbm8a group exhibited a significant decrease in the number of Aβ-associated DNs, including ATG9A+ DNs (P < 0.01; Figure 3B) and LC3B+ DNs (P < 0.05; Figure 3E). Furthermore, the ratio of 6E10+GFP+ATG9A+/GFP+6E10+ (P < 0.05; Figure 3C) or 6E10+GFP+LC3B+/GFP+6E10+ (P < 0.05; Figure 3F) was significantly reduced in the 5×FAD-Rbm8a group relative to the 5×FAD-Ctrl group. These findings indicate that Rbm8a can significantly inhibit the formation of DNs, offering a promising treatment option to reduce neuronal dysfunction and loss in AD.
Figure 4| Reduced gliosis response of astrocytes and microglia in the DG of 5×FAD mice following Rbm8a overexpression.
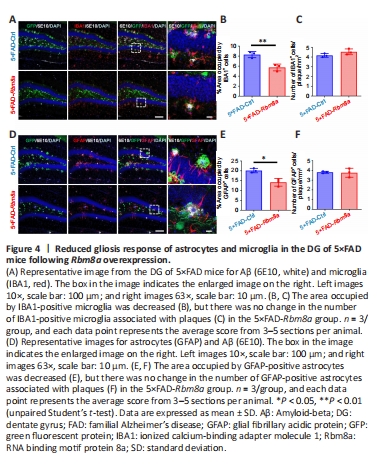
Accumulating evidence suggests that glial cells can engulf and aggregate plaques, thereby reducing local plaque-associated toxicity (Sosna et al., 2018). Therefore, the effect of Rbm8a on gliosis and the phagocytic activity of surrounding glial cells towards amyloid plaques were explored. Immunofluorescence staining was used to detect microglia distribution and gliosis reaction (Figure 4A), revealing a decreased area occupied by IBA1+ microglia (P < 0.01; Figure 4B) in the DG region of the 5×FAD-Rbm8a group compared with the 5×FAD-Ctrl group, while no significant difference in the average number of microglia surrounding each plaque was observed (P > 0.05; Figure 4C). Furthermore, the astrocyte-occupied area in the 5×FAD-Rbm8a group was reduced (P < 0.05; Figure 4D and E), but no significant change was found in the average number of astrocytes surrounding each plaque (P > 0.05; Figure 4F), compared with the 5×FAD-Ctrl group. Overall, these results suggested that Rbm8a suppressed the gliosis response of glial cells without affecting their phagocytic function towards amyloid plaques.
Figure 6|Rbm8a overexpression increases the number of immature neurons in the DG of 5×FAD mouse brain.

In addition to plaque formation in the DG, neuron loss also contributes to memory impairment in AD. However, Rbm8a has been shown to play a critical role in neural progenitor cell proliferation (Al Mamun et al., 2022). Therefore, whether Rbm8a overexpression in the DG can lead to an increase in neuronal number is of great interest. This study found that the number of immature neurons (DCX+) and the percentage of DCX+GFP+ cells relative to GFP+ cells in the DG were significantly increased in 5×FAD-Rbm8a mice compared with 5×FAD-Ctrl mice (P < 0.01; Figure 6A–C). Moreover, the relative expression levels of DCX mRNA showed a significant increase in 5×FAD-Rbm8a mice compared with the 5×FAD-Ctrl group mice (P < 0.01; Figure 6D). This indicates that Rbm8a can stimulate an increase in the number of immature neurons in AD.
Figure 7|Rbm8a overexpression promotes neurogenesis in the DG of 5xFAD mouse brain.

Immature neurons develop from stem cells, and an increased number indicates neurogenesis in the DG. An experiment was conducted (Figure 7A) to determine the neurogenesis stage in which Rbm8a is involved (the process of neurogenesis is described by a schematic diagram [Figure 7B]). Anti-GFAP-targeted radial glial stem cells were not infected by the virus (Figure 7C and D), which indicated that Rbm8a overexpression did not affect this stage in neurogenesis. In other neurogenesis stages, a significant increase in the number of SOX2+ cells (P < 0.05; Figure 7E and F) and DCX+ cells (P < 0.05; Figure 7H and I) were observed, as well as an increase in the percentage of SOX2+GFP+/GFP+ cells (P < 0.01; Figure 7G) and percentage of DCX+GFP+/GFP+ cells (P < 0.001; Figure 7J). These results suggested that the increase in immature neurons was due to increased neural progenitor cells induced by Rbm8a overexpression in the DG.
Figure 8|Increased proliferation of neural progenitor cells induced by Rbm8a overexpression in the DG.
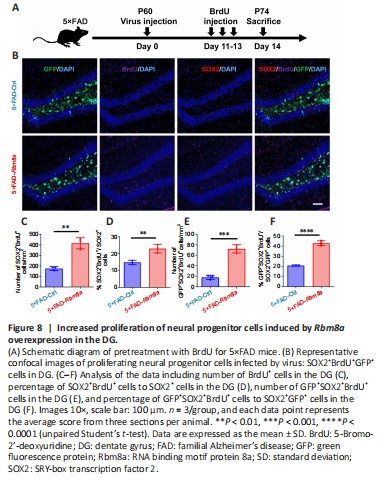
During neurogenesis, neural stem cells and progenitor cells proliferate and differentiate into newborn neurons (Lindsey et al., 2018). The upregulation of Rbm8a in the DG was hypothesized to induce self-proliferation of neural progenitor cells, resulting in increased cell numbers. To confirm this hypothesis, the experiment illustrated in the schematic diagram was performed (Figure 8A). Immunofluorescence staining using anti-BrdU and anti-SOX2 (Figure 8B) revealed a significant rise in the quantity of SOX2+BrdU+ cells (P < 0.01; Figure 8C), as well as an increase in the proportion of SOX2+BrdU+/SOX2+ cells (P < 0.01; Figure 8D) and BrdU+GFP+SOX2+ cells (P < 0.001; Figure 8E). In addition, a significantly greater percentage of GFP+SOX2+BrdU+/SOX2+GFP+ cells was observed in the DG region of the 5×FAD-Rbm8a group mice (P < 0.0001; Figure 8F). These data indicated that Rbm8a promotes the proliferation of neural progenitor cells, which may further lead to an increase in immature neuronal cell density in the DG region.