脑损伤
-
Figure 3|The effects of delayed overexpression of cerebral Ras-related C3 botulinum toxin substrate (Rac) 1 on functional recovery after ischemic stroke in aged mice.
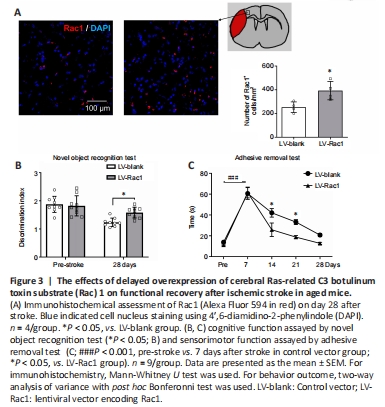
To assess whether overexpression of cerebral Rac1 could improve post-stroke functional recovery in aged mice, lentiviral vectors carrying Rac1 were administrated into mice brain on day 1 after MCAO. We confirmed the effect of the vector by demonstrating an increased number of Rac1-positive cells 27 days after injection compared with the control group (P < 0.05; Figure 3A). Cerebral ischemia produced the impairment of cognitive function and sensorimotor function (Bu et al., 2020). Herein, delayed overexpression of Rac1 improved cognitive recovery on day 28 (P < 0.05; Figure 3B). In addition, the sensorimotor function was assayed. MCAO caused a marked impairment assessed by adhesive removal test (pre-stroke vs. 7 days after stroke in the control group, P < 0.05). Importantly, LV-Rac1 also produced an improved performance in sensorimotor function recovery compared with the control group (P < 0.05 on days 14 and 21 after stroke; Figure 3C).
Figure 5|The effect of delayed overexpression of cerebral Ras-related C3 botulinum toxin substrate (Rac) 1 on neurite density, endothelial proliferation, and tissue loss on day 28 after stroke in aged mice.
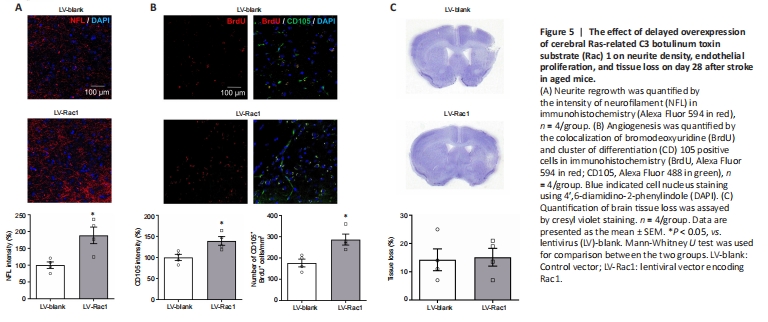
Our previous study showed that cell-specific overexpression of neuronal Rac1 promoted axonal regeneration and that overexpression of endothelial Rac1 promoted angiogenesis in the young mouse brain after stroke (Bu et al., 2019, 2020). In the present study, we examined the role of cerebral Rac1 in these two major forms of plasticity in aged mice. We found that delayed overexpression of cerebral Rac1 improved neurite outgrowth (P < 0.05; Figure 5A) in the peri-infarct zone compared with the control group on day 28 after stroke. In addition, endothelial proliferation was also improved by delayed overexpression of cerebral Rac1 as evidenced by the increase of either CD105 staining intensity (P < 0.05; Figure 5B) or the number of CD105 positive cells with BrdU (P < 0.05; Figure 5B) in the peri-infarct zone compared with the control group on day 28 after stroke. We further performed CV staining to evaluate the tissue loss. Compared with the control group, no differences in cavity sizes were seen after treatment (P > 0.05; Figure 5C).
Figure 6|The effect of delayed inhibition of Ras-related C3 botulinum toxin substrate 1 on neurite density, endothelial proliferation, and tissue loss on day 28 after stroke in aged mice.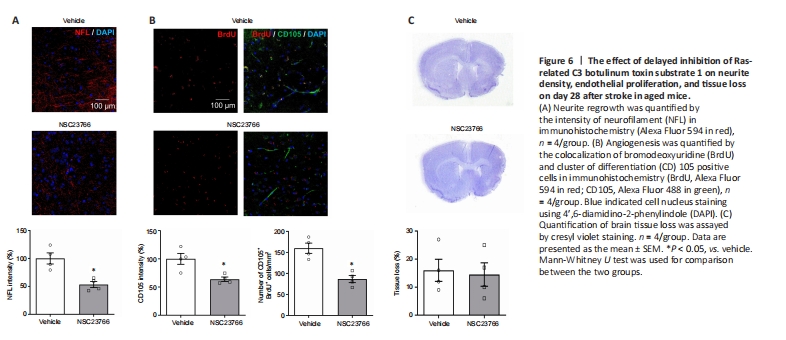
The contribution of cerebral Rac1 to axonal density and endothelial proliferation was confirmed using Rac1 inhibitor NSC23766. We found that delayed inhibition of Rac1 reduced NFL intensity (P < 0.05; Figure 6A), CD105 intensity (P < 0.05; Figure 6B), and the number of proliferative endothelial cells (P < 0.05; Figure 6B) compared with the control group on day 28 after stroke. No differences in tissue loss assessed by CV staining were observed after treatment (P > 0.05; Figure 6C).