周围神经损伤
-
Figure 1|Characterization of PLGA@Col/HA conduits.
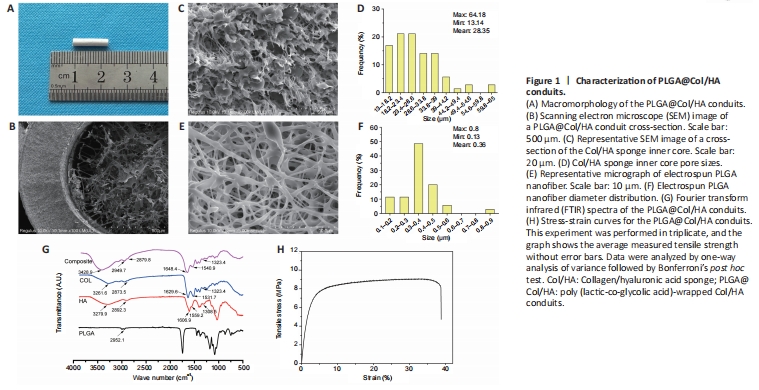
The nerve conduit had a tubular structure with an outer diameter of 2.4 mm and an inner diameter of 1.2 mm, the tube wall was 0.6 mm thick, and the total length used for transplantation was 12 mm (Figure 1A). The scanning electron micrographs showed that the microporous inner Col/HA sponge and the outer electrospun PLGA nanofibrous layer formed a tubular lumen, which could provide a guide channel for axon regeneration (Figure 1B). As shown in Figure 1C, the lyophilized inner Col/HA sponge exhibited an interconnected porous microstructure, which could facilitate nutrient and metabolite transport. The pore sizes in the PLGA@Col/HA conduit ranged from 13.14 to 64.18 μm, and 77% of the pores were 18.2–49.4 μm in diameter (Figure 1D). The diameter of the electrospun PLGA nanofiber ranged from 0.13 to 0.80 μm, and 68% of the PLGA@Col/HA conduit exhibited a diameter of 0.3–0.5 μm (Figure 1E and F). To ascertain the secondary structure of the PLGA@Col/HA conduit, chemical analysis was performed by FTIR spectroscopy. Characteristic C-H (2952.1 and 2949.7 cm–1), amide A (3279.9, 3261.6, and 3428.9 cm–1), amide B (2892.3, 2879.8, and 2873.5 cm–1), amide I (1606.9, 1629.6, and 1648.4 cm–1), amide II (1559.2, 1531.7, and 1540.9 cm–1), and amide III (1308.5, 1323.4, and 1343.4 cm–1) peaks were observed, indicating CH2 and CH3 groups in Col/HA and C-H and C=O bonds in PLGA, showing that Col and HA were preserved in the final conduits (Figure 1G). Analysis of the mechanical properties of the conduits showed that the ultimate tensile strength of the PLGA@Col/HA conduit was 9.47 ± 0.29 MPa, the elongation at break was 44.41 ± 3.23%, and the tensile modulus was 296.43 ± 109.25 MPa (Figure 1H). These results indicate that the PLGA shell provides sufficient mechanical support for the Col/HA sponge core.
Figure 2|Identification of hUCMSCs and hUCMSC-derived exosomes.
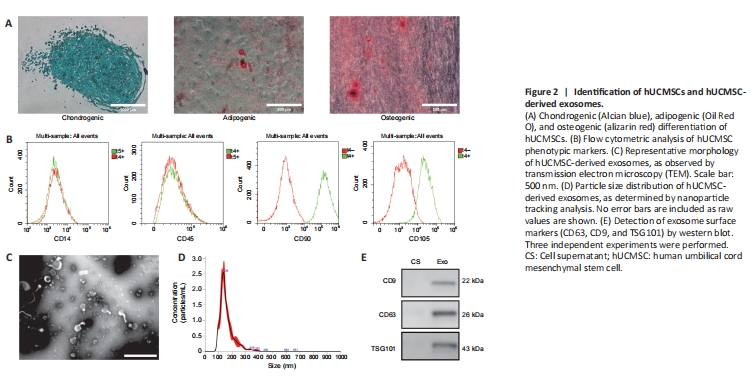
To assess the ability of hUCMSCs to differentiate into multiple lineages, chondrocytes, adipocytes, and osteocytes differentiated from hUCMSCs were detected by staining with Alcian blue, Oil Red O, and alizarin red, respectively (Figure 2A). Flow cytometry analysis detected expression of the MSC phenotypic markers CD90 and CD105, whereas expression of the hematopoietic phenotypic markers CD14 and CD45 was not detected (Figure 2B). Taken together, these findings confirmed that the cells used in this experiment were human umbilical cord MSCs. TEM imaging showed that purified human umbilical cord MSC-derived exosomes exhibited a cup-shaped morphology (Figure 2C). Furthermore, nanoparticle tracking analysis (NTA) showed that the average diameter of the exosomes was 159.9 ± 56.7 nm, and the peak diameter was 136 nm (Figure 2D). To further investigate the specific surface markers of the exosomes, we performed western blot analysis, which indicated that the hUCMSC-derived exosomes expressed the exosome-specific markers CD63, CD9, and TSG101 (Figure 2E). These results indicate that we successfully isolated human umbilical cord MSCs and their corresponding exosomes.
Figure 3|Effect of hUCMSC-derived exosomes on neurite growth.
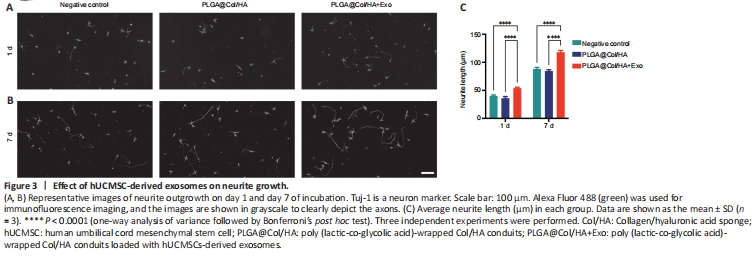
Next, neurons were co-cultured with different conduits to explore the effects of the conduits and hUCMSC-derived exosomes on neurite outgrowth. Immunofluorescence staining (Tuj-1) was performed to examine the effect of hUCMSC-derived exosomes on neurites (Figure 3A and B). We found that neurites were longer on day 7 compared with day 1 of co-culture. The average neurite length of neurons incubated with hUCMSC-derived exosomes was significantly greater compared with the negative control and non-exosome group on day 1 and day 7 (P < 0.0001; Figure 3C). These results suggest that the hUCMSC-derived exosomes promoted neurite elongation within 24 hours. There was no significant difference in neurite growth between the negative control group and the PLGA@Col/HA group, indicating that the conduits are not toxic to cells (P > 0.05).
Figure 5|Effect of PLGA@Col/HA+Exo on gastrocnemius muscle atrophy in rats with nerve defects.
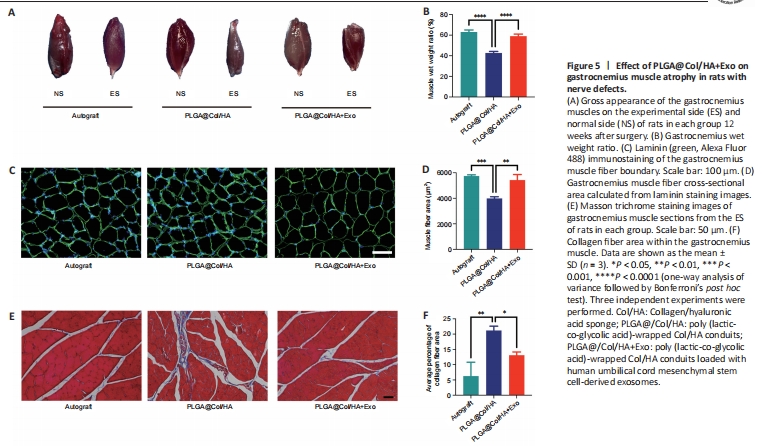
After sciatic nerve injury, contraction of the denervated muscles becomes difficult, and this typically leads to chronic muscle atrophy. Although the gastrocnemius muscles in each group exhibited atrophy, the degree of atrophy improved over time in the PLGA@Col/HA + Exo group. The gastrocnemius muscles were harvested from both legs of rats in each group after the electrophysiological testing was complete. Next, the gastrocnemius muscles were weighed and photographed (Figure 5A). The ES gastrocnemius was visibly smaller than the NS gastrocnemius muscle in rats from the PLGA@Col/HA group, and this size difference was even more pronounced in the other two groups. There was no significant difference in the wet weight ratio between the PLGA@Col/HA + Exo group and the autograft group (P = 0.0855). However, the gastrocnemius wet weight ratio was significantly greater in the PLGA@Col/HA + Exo group than in the PLGA@Col/HA group (P < 0.0001; Figure 5B). As shown in Figure 5C and D, there was no significant difference in average cross-sectional muscle fiber area between the PLGA@Col/HA + Exo group and the autograft group (P = 0.3739), and the values for both were significantly higher than that of the PLGA@Col/HA group (P < 0.0014). Abnormal collagen fiber deposition around the muscle implies decreased motor function. Masson trichrome staining of muscle cross-sections demonstrated widespread collagen deposition around atrophied muscle fibers in the PLGA@Col/HA group (Figure 5E). The average percentage of collagen area in the PLGA@Col/HA + Exo group and the autograft group was significantly smaller than that in the PLGA@Col/HA group (Figure 5F). These results indicate that treatment with hUCMSC-derived exosomes reduced abnormal deposition of collagen fibers in rats, which is consistent with the motor function recovery results.
Figure 6|Effect of PLGA@Col/HA+Exo on axonal regeneration in rats with nerve defects.
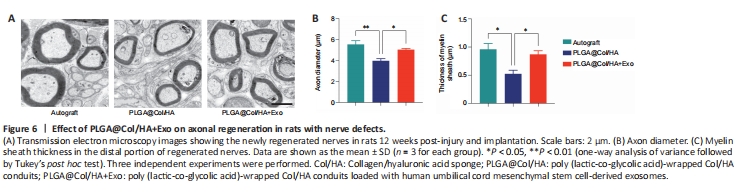
Twelve weeks after surgery, the axon diameter and myelin sheath thickness of regenerated sciatic nerve fibers were examined by transmission electron microscopy (Figure 6A). In the transverse sections, clusters of small-diameter unmyelinated nerve bundles were seen around the myelinated axons, and the regenerated myelinated axons were surrounded by distinct layers of myelin sheath. In the autograft group and the PLGA@Col/HA + Exo group, the regenerated axons were wrapped in clear, thick myelin sheaths. Compared with the PLGA@Col/HA group, the diameters of the axons were significantly higher in the PLGA@Col/HA + Exo group (P = 0.046; Figure 6B). As shown in Figure 6C, the myelin sheaths were thicker in the PLGA@Col/HA + Exo group than in the PLGA@Col/HA group (P = 0.0483). The structure of the regenerated myelinated axons in the PLGA@Col/HA + Exo group was similar to that in the autograft group. There were no significant differences between the autograft group and the PLGA@Col/HA + Exo group in terms of either myelin thickness or diameter (P > 0.05). These findings confirm that the efficacy of the PLGA@Col/HA conduit loaded with hUCMSC-derived exosomes is close to that of standard autograft in promoting axonal and myelin regeneration.
Figure 7|Effect of PLGA@Col/HA+Exo on neurovascular regeneration in rats with sciatic nerve injury.
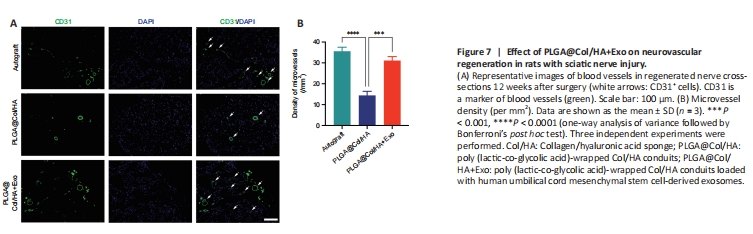
To evaluate angiogenesis in each group, immunofluorescent staining of cross-sections of the regenerated nerves with an anti-CD31 antibody was performed. In Figure 7A, the green areas indicated by white arrows are CD31+ cells, and the green closed circles are blood vessels. The microvessel density for each group was calculated based on the immunofluorescence results (Figure 7B). The microvessel density was greater in the PLGA@Col/HA + Exo and autograft groups than in the PLGA@Col/HA group (P < 0.0001), and there was no significant difference in microvessel density between the PLGA@Col/HA + Exo group and the autograft group (P = 0.0686). Thus, the 3D composite conduit loaded with hUCMSC-derived exosomes promoted recovery after peripheral nerve injury by improving angiogenesis and vascularization, which mediate oxygen and nutrient transport.
Figure 8|Histopathological changes in rats tissues 12 weeks after transplantation.
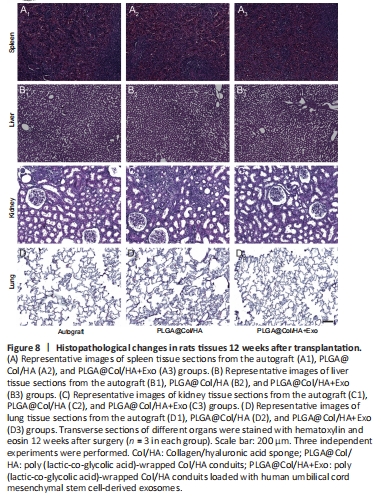
To verify the safety of hUCMSC-derived exosomes and PLGA@Col/HA conduits in vivo, with a view to clinical use in the future, 12 weeks after transplantation we harvested and stained sections of major rat organs. We selected organs that are prone to tumorigenesis or toxic injury, namely the spleen, liver, kidney, and lung, and subjected them to hematoxylin and eosin staining. As shown in Figure 8A, spleen sections from all groups showed normal structure. No sinus dilatation, necrosis, or inflammation were seen in the liver sections (Figure 8B). Likewise, there were no histopathological findings of congestion, necrosis, or vascular dilation in the kidney sections from all groups (Figure 8C). As expected, no tumors or abnormal nodules were seen in lung sections from any of the groups, which was probably avoided by the application of small local doses of exosomes (Figure 8D). In summary, no morphological abnormalities were found in these tissues, indicating that the hUCMSC-derived exosomes and PLGA@Col/HA conduits did not induce any detectable toxicity or tumor formation.