神经退行性病
-
Figure 1| The modified dual SMAD pathway inhibition protocol.
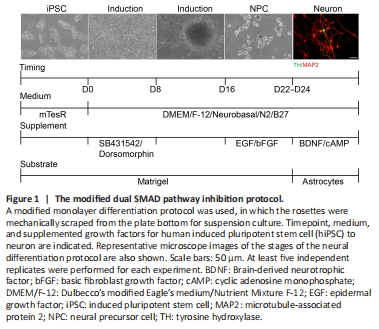
Based on a previous study (Chambers et al., 2009), we used 5 μM dorsomorphin and 5 μM SB431542 in an early induction culture system from day 0 to day 8 to promote neural differentiation in hiPSC. This showed that hiPSC morphology started to become organized columnar epithelial cells after the addition of both inhibitors. On day 9, we found that the cell clusters gradually showed NPC characteristics. On day 16, densely adherent cell tissue was mechanically separated into homogeneous cell clusters by a scraper approach. These cell clusters were then cultured in a suspension system that included EGF and FGF. NPC were passaged for three generations and then cultured with an astrocyte feeder for further differentiation into neurons, which were cultured with BDNF and c-AMP. The results showed that this modified protocol could successfully induce hiPSC into neurons after 24 days (Figure 1). This protocol was named the dual SMAD pathway inhibition protocol.
Figure 2| The modified dual SMAD pathway inhibition protocol yielded a low proportion of dopaminergic neurons.

As shown, hiPSC expressed OCT4 and SSEA4 before differentiation, reflecting the stem cell characteristics and multi-differentiation potential of the hiPSC in our study (Figure 2A). The morphology of hiPSC changed during culture from day 0 to day 8 with dual inhibitor. On day 20, both SOX2 and Nestin were highly expressed, indicating that cells at this stage showed NPC characteristics (Figure 2B). Overall, 84% of cells successfully differentiated into neurons, based on MAP2 immunofluorescence staining (Figure 2C and D). On day 33, we observed the expression of multiple subtypes of neuronal markers such as dopaminergic neurons (Figure 2D). The results showed that different subtypes of neurons including 13% glutaminergic neurons, 21% dopaminergic neurons, and 56% GABAergic neurons (Figure 2E and F). However, few cells expressed mDA biomarkers.
Figure 3| The SHH + FGF8 differentiation protocol.

An increasing number of studies have shown that the application of growth factors or certain small molecules (such as smoothened agonists, SHH, TGF-β3, FGF8, retinoic acid, LIF, stromal cell-derived factor 1a) are beneficial to the induction of dopaminergic neurons (Storch et al., 2001; Guyon et al., 2006; Katsuki et al., 2009; Schwartz et al., 2012; Blaess and Ang, 2015). Considering that SHH and FGF8 can control the fate of dopaminergic neurons along the D-V and A-P axes, cooperating to specify dopaminergic neurons, we added SHH and FGF8 into the culture medium from day 8 to day 22. For better survival of dopaminergic neurons, supplemental addition of 20 ng/mL GDNF and 0.2 mM AA was essential for neuronal differentiation. After the addition of SHH and FGF8, hiPSC were successfully differentiated into neurons with a higher ratio of dopaminergic neurons after 24 days (Figure 3). This optimized protocol was named the SHH + FGF8 differentiation protocol.
Figure 4| The SHH + FGF8 differentiation protocol yielded a high proportion of dopaminergic neurons, with a small fraction of neurons that expressed FOXA2, a marker of midbrain dopaminergic neurons.

On day 20 of the SHH + FGF8 differentiation protocol, induced cell clusters in suspension culture showed neurosphere-like characteristics, and the neurospheres were Nestin- and SOX2-positive (data not shown), indicating that these neurosphere cells had NPC characteristics. Further, NPC at this stage expressed FOXA2 and SOX2, indicating that these cells also have FP progenitor properties (Figure 4A). Immunofluorescence staining of cells on day 33, showed that MAP2-positive neurons accounted for 83% of the total induced cells and TH-positive cells for 83%, which is similar to the SMAD protocol (Figure 4B and C). Moreover, application of SHH and FGF8, significantly increased the proportion of dopaminergic neurons to approximately 75% of the total differentiated neurons, compared with only 21% in the previous protocol (Figure 4D and E). To verify whether differentiated TH-positive neurons have the potential to develop to the maturation stage, we transfected single cells after NPC digestion with a DAT-Cherry lentivirus on day 22, and then examined expression of TH by immunofluorescence staining. Co-expressed DAT-Cherry and TH neurons were observed on day 33 in neural induction cultures (Figure 4F), suggesting that induced dopaminergic neurons partially show early maturation properties. In addition, we also confirmed that a small number of these dopaminergic neurons were positive for the midbrain marker, FOXA2 (Figure 4G). This indicates that the SHH + FGF8 protocol not only greatly improved the differentiation efficiency of dopaminergic neurons, but also benefited the induction of mDA neurons. It is known that mDA neurons play an important role in cognitive, emotional, and neurodegenerative disorders. Thus, there is a need to further optimize the induction protocol to increase the differentiation efficiency of mDA neurons.
Figure 5| The CHIR + SHH + FGF8 induction protocol.

Previous studies have shown that CHIR99021 (Kriks et al., 2011), a potent GSK3β inhibitor, can strongly activate WNT signaling and promote the differentiation of floor plate progenitors, which is particularly important for the development of mDA neurons. The addition of Purmorphamine in combination with SHH (a small molecule agonist) activates SHH signaling (Fasano et al., 2010). Thus, we further improved the induction protocol by directly adding 0.7 μM CHIR99021 and 2 μM Purmorphamine at the early stage of the SHH + FGF8 differentiation protocol. However, no NPC-like or neuron-like cells were observed throughout the entire induction course. We next modified the protocol by adding SHH, FGF8, and Purmorphamine into the neuronal induction system from day 1 to day 7 of hiPSC induction, with 0.7 μM CHIR99021 added from day 3 to day 13. This allowed us obtain a high proportion of mDA neurons (Figure 5). This protocol was named the CHIR + SHH + FGF8 induction protocol.
Figure 6| The CHIR + SHH + FGF8 induction protocol more effectively induced hiPSC differentiation into mDA neurons.
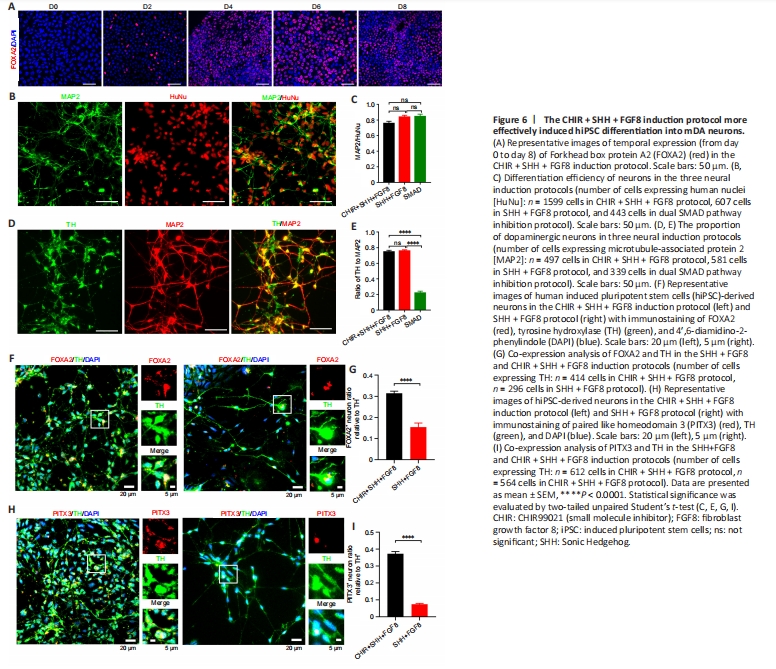
The CHIR + SHH + FGF8 induction protocol showed that approximately 80% of the induced cells expressed FOXA2, a key marker of mDA neuronal development, in the early stage of differentiation on day 6 (Figure 6A). We found no statistical difference in neural differentiation efficiency between the CHIR + SHH + FGF8 induction protocol and SHH + FGF8 differentiation protocol (Figure 6B and C). However, the percentage of differentiated dopaminergic neurons was significantly higher (74%) with the addition of CHIR99021, compared with the dual SMAD pathway inhibition protocol (Figure 6D and E). We also found that more dopaminergic neurons were co-labeled with midbrain markers such as FOXA2 and PITX3 on day 41 under the CHIR + SHH + FGF8 induction condition, compared with the SHH+FGF8 differentiation protocol (Figure 6F–I). We found that about 31% of TH-positive neurons induced in this protocol were co-labeled with FOXA2, whereas only 15% were co-labeled in the SHH + FGF8 differentiation protocol (Figure 6F and G). About 37% of TH-positive neurons in this protocol were co-labeled with PITX3, whereas only 7% were co-labeled in the SHH + FGF8 differentiation protocol (Figure 6H and I). Of particular note, 80% of the cells expressed FOXA2-formed FP progenitors by day 6 of cell culture in the CHIR + SHH + FGF8 induction protocol. On day 41, approximately 31% of TH-positive neurons were co-labeled with FOXA2 and approximately 37% of TH-positive neurons were co-labeled with PITX3 (Figure 6G and I). FOXA2 is critical for determining the ventral midbrain identity of neurons, but not all mDA neurons express FOXA2 after maturation of mDA neurons (Kirkeby et al., 2017; Yeap et al., 2023). In addition, PITX3 is critical for the precise induction of mDA neuronal maturation and is more highly expressed in mature mDA neurons (Kouwenhoven et al., 2017). Therefore, neither FOXA2 nor PITX3 alone is sufficient to define all mDA neurons. Experimental data in this study showed that the percentage of TH-positive neurons was approximately 74%, therefore we speculated that the percentage of mDA neurons induced by the CHIR + SHH + FGF8 induction protocol varies between 31–74%.
Figure 7|Electrophysiological properties of dopaminergic neurons derived from the CHIR + SHH + FGF8 induction protocol.
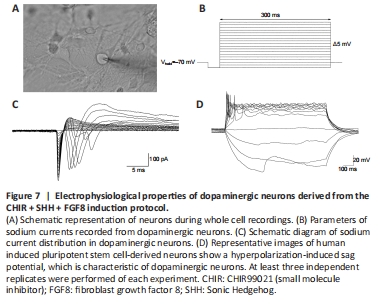
We then performed electrophysiological tests (Figure 7A) on the neurons induced by our neural induction protocol. The results showed that the neurons exhibited sodium currents when the voltage was held at –70 mV (Figure 7B and C), and a hyperpolarization-induced “sag” potential characteristic when action potentials were evoked (Figure 7D); this is consistent with characteristics of dopaminergic neuronal subtypes (Yang et al., 2001; Niclis et al., 2017; Zou et al., 2018). Therefore, our evidence suggests that the application of SHH, FGF8, and Purmorphamine combined with CHIR99021 during neural induction improved the efficiency of mDA neuronal differentiation. This offers the possibility of generating a large number of mDA neurons for translational medicine research.