神经退行性病
-
Figure 1|Overexpression of tau-N368 fragment inactivated STAT3 by inhibiting nuclear translocation.

Our earlier research showed that accumulation of htau or P301L-htau reduced STAT3 activity (Hong et al., 2020; Wan et al., 2021). AEP-derived tau fragments (tau-N368) are prone to aggregation and strongly trigger neurodegeneration (Zhang et al., 2014; Wang et al., 2018a). We thus speculated that tau-N368 accumulation may influence the activity of STAT3. On the basis of previous studies, the cleaved tau1–368 band was identified using two distinct tau antibodies (HT7 and tau5) (Additional Figure 2), with tau-N368 expression detected using the anti-HT7 antibody. Consistent with our earlier research (Hong et al., 2020; Wan et al., 2021), after transfection of the tau-N368 plasmid into HEK293 cells (Figure 1A and B), or AAV-eGFP-tau-N368 virus injection into the hippocampal CA3 region of C57BL/6 mice (Figure 1C and D), we found that overexpressing tau-N368 increased the levels of STAT3 phosphorylation at Tyr705 (pY705), while total STAT3 levels showed no change (Figure 1A and C). However, the nuclear fraction showed a reduction in both total STAT3 and its phosphorylation at Ser727 (pS727), which positively correlated with the transcriptional activity of STAT3 after it shifted subcellular localization to the nucleus (Figure 1B and D). We used immunofluorescence to show that increased levels of tau-N368 lowered nuclear STAT3 levels (Figure 1E). EMSA using an oligonucleotide probe bearing the STAT3 binding domain found that an increase in tau-N368 levels inhibited the capacity of STAT3 to bind to DNA, and the use of a cold probe disrupted this association (Figure 1F). Furthermore, a TF luciferase experiment confirmed that tau-N368 overexpression attenuated STAT3 activity (Figure 1G). These results demonstrate that an increase in intracellular tau-N368 led to suppression of STAT3 activity.
Figure 2|Overexpression of STAT3 ameliorated tau-N368-induced cognitive deficits.

Figure 3|Overexpressing STAT3 ameliorated tau-N368-induced memory deficits.
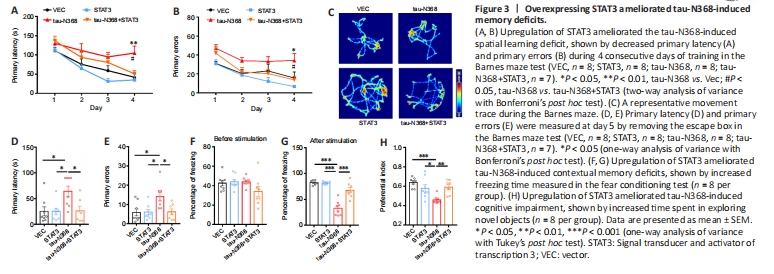
As STAT3 overexpression attenuated htau/P301L-tau induced behavior disorders (Hong et al., 2020; Wan et al., 2021), we examined the influence of STAT3 overexpression to gain knowledge about the involvement of STAT3 in tau-N368-induced memory and learning impairments. Two-month-old C57BL/6 mice received bilateral injections of AAV-STAT3 mixed with AAV-tau-N368 into the CA3 region of the hippocampus. STAT3 overexpression was confirmed after 1 month by western blotting (Figure 2A) and immunofluorescence (Figure 2B). We investigated how activation of STAT3 affected spatial learning and memory using the Morris water maze experiment. On days 4–6, learning ability was hampered due to tau-N368 overexpression, shown by a markedly increased time to locate the hidden platform. Increasing STAT3 expression on days 5 and 6 prevented these tau-N368-induced learning impairments (Figure 2C). In memory testing on day 7, we found that STAT3 and tau-N368 overexpressing mice showed a reduced average latency in arriving at the previous target quadrant, more platform crossings, and remained for a prolonged period in the desired quadrant compared with tau-N368 mice (Figure 2D–G). No remarkable difference was observed in swimming velocity across the groups, indicating that motor impairments did not account for the findings (Figure 2H). Mice were tested for their spatial learning and memory using the Barnes maze, which is a behavioral model based on a dry-land environment (Pitts, 2018). In the Barnes maze, tau-N368 mice displayed a significant increase in primary latency and primary errors to entering the escape hole on day 4 of the training phase. Overexpressing STAT3 rescued the cognitive deficits induced by tau-N368 (Figure 3A and B). Throughout the probe phase on day 5, we saw that mice overexpressing STAT3 and tau-N368 more frequently entered the exit quadrant (where the escape box was previously hidden) than tau-N368 mice (Figure 3C). Similarly, overexpressing STAT3 improved tau-N368-induced memory deficits, shown by reduced primary latency and primary errors to enter the escape hole (Figure 3D and E). This enhanced long-term memory was further demonstrated by an increase in the freezing period during the memory test of the fear conditioning test (Figure 3F and G). Furthermore, using a novel object recognition test, mice overexpressing STAT3 and tau-N368 spent more time investigating the new object compared with tau-N368 mice (Figure 3H). Overall, these results suggested that activation of STAT3 ameliorated tau-N368-induced cognitive and memory deficits.
Figure 6|Overexpression of STAT3 ameliorated tau-N368-induced neuronal loss.
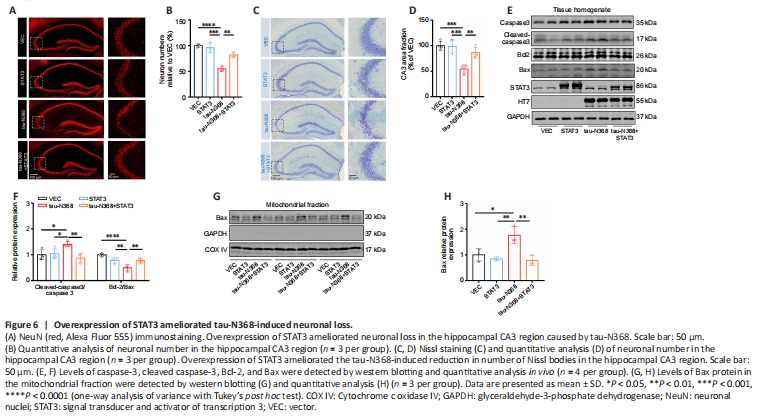
In contrast with wild-type or other tau fragments, tau-N368 induced substantial apoptosis (Zhang et al., 2014). Using HEK293 cells, we transfected tau-N368 and STAT3 plasmids to determine if STAT3 is implicated in apoptosis triggered by tau-N368. Overexpression of tau-N368 significantly decreased cell survival compared with controls. However, overexpression of STAT3 attenuated tau-N368-induced cell cytotoxicity (Figure 5A and B). Western blotting revealed that STAT3 overexpression reduced the increase of cleaved caspase-3 levels caused by tau-N368 in vitro (Figure 5C and D). A common pathogenic phenotype of AD is neuronal death (Deshpande et al., 2019; Jayaraman et al., 2021; Yepes, 2021). As expected, STAT3 overexpression reversed tau-N368-induced neuronal loss in the hippocampal CA3 region by immunofluorescence, Nissl staining, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (Additional file 1, Additional Figure 3, and Figure 6A–D). We also found that STAT3 overexpression ameliorated neuronal apoptosis by decreasing Bax protein levels in AAV-tau-N368 mice (Figure 6E and F). We also examined Bax protein levels in the mitochondrial fraction, and found that overexpressing STAT3 reversed the tau-N368-induced increase in total Bax levels in the mitochondrial fraction (Figure 6G and H).