脑损伤
-
Figure 1|Effect of BNN-20 on neuronal differentiation of human iPSC-derived NPCs.
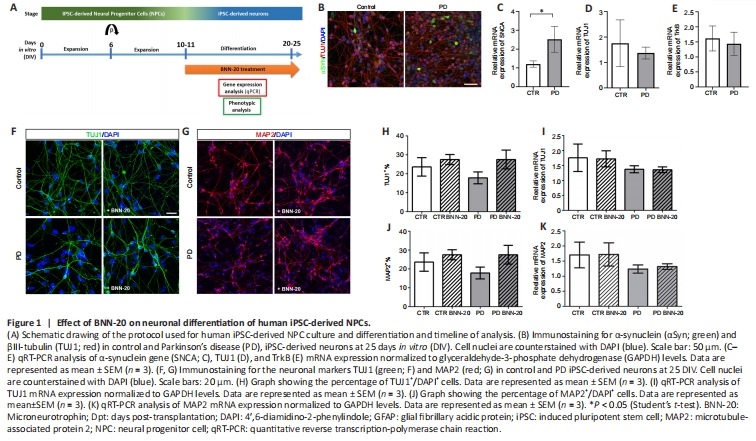
Human NPCs are a potential source of cells for CRTs, but also a valid experimental system to model PD; thus, we investigated the effects of BNN-20 on these cells derived either from healthy or parkinsonian donors carrying the p.A53T α-synuclein mutation (G209A in the SNCA gene) (Kouroupi et al., 2017). Both control and PD iPSC-derived NPC cultures expressed Nestin and Pax6 with no significant differences between them (data not shown; Kouroupi et al., 2017). NPCs were allowed to differentiate spontaneously into βΙΙΙ-tubulin-expressing (TUJ1+) neurons for two weeks (Figure 1A, B, and D). α-Synuclein (αSyn) protein was present in the soma and neurites of both PD and control iPSC-derived neurons, though more cells were strongly positive for αSyn in PD cultures (Figure 1Β) and levels of αSyn mRNA were significantly higher in PD cultures (P < 0.05; Figure 1C).
After confirming the expression of TrkB neurotrophin receptor, through which BNN-20 acts in vivo (Botsakis et al., 2017; Figure 1E), we cultured control and PD iPSC-derived NPCs under spontaneous neuronal differentiation conditions, with or without BNN-20, for up to 25 DIV (Figure 1A). Although Qrt-PCR analysis did not reveal significant differences between control, PD and BNN-20-treated cultures, slightly increased, but not statistically significant, number of TUJ1+ neurons was observed in PD cultures after BNN-20 treatment (P = 0.056; Figure 1F–K).
Figure 2|Effect of BNN-20 on PD-associated phenotypes.
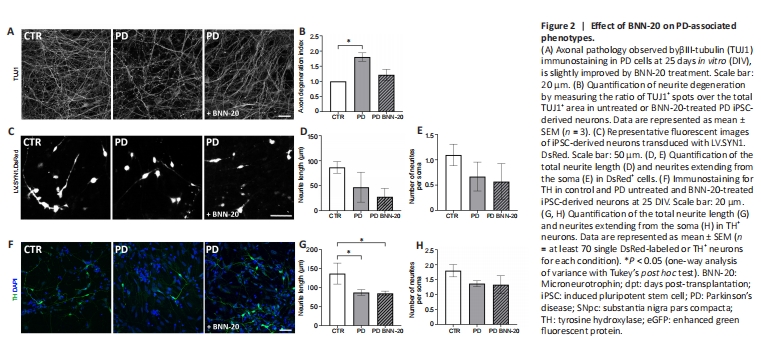
Subsequently, we assessed the effects of BNN-20 on disease-associated phenotypes of PD cultures. Upon differentiation, PD iPSC-derived neurons exhibit degeneration traits exemplified by contorted or fragmented TUJ1+ neurites, displaying αSyn+ swollen varicosities and large spheroid inclusions, similar to the dystrophic neurites of p.A53T patients (Duda et al., 2002; Kouroupi et al., 2017). Here, we identified TUJ1+ fragmented axons at the second week of neuronal differentiation (NDI, Control vs. PD, P=0.019; Figure 2A and B). Moreover, after lentiviral expression of DsRed in a concentration enabling us to label single neurons, we found that the average neurite length was reduced -but not significantly- in PD neurons (Control vs. PD, P = 0.098; Figure 2C and D) as was the total number of neurites extending from the soma (Control vs. PD, P = 0.106; Figure 2E). Neurons differentiated in the presence of BNN-20 were partially protected, particularly with respect to degeneration as their DI was not significantly different from that of control cells (P < 0.05; Figure 2B). Yet, impaired neurite length and branching were not restored by BNN-20 in either the neuronal population (Figure 2C–E) or, more specifically, in TH+ dopaminergic neurons (Figure 2F–H; control vs. PD, P < 0.05; one-way analysis of variance).
Figure 3|Evaluation of the viability and differentiation of the grafted cells, and of the effect of BNN-20 + graft treatment on the “weaver” substantia nigra.

Next, we performed unilateral grafts of 200.000 NPCseGFP just above the SNpc of P45 wild-type mice and killed the animals 3 days post-transplantation (dpt) to check successful graft targeting. We used the contralateral side as internal control and we observed eGFP+ cells only at the intended area, in the dorsal margin of the SNpc. A fraction of eGFP+ cells had already started to differentiate towards the neuronal or the astroglial lineage, co-expressing Dcx or GFAP, respectively (Figure 3A and data not shown).
We performed similar transplantations in P45 “weaver” mice that had received BNN-20 or vehicle for 15 days (from P30 to P45) and we continued the BNN-20/vehicle administration for another 15 days. The mice were killed at P60, or at P75 (15 and 30 dpt, respectively) (Figure 3B and C). Before looking at the grafts, we assessed the progress of dopaminergic degeneration, focusing on the contralateral, non-grafted, hemispheres of control mice (that received vehicle) (Figure 3D) and we found that the numbers of TH+ cells at P75 were almost 1/3rd of those at P60, even though this decrease did not reach statistical significance. This ongoing degeneration was not inhibited or ameliorated by BNN-20 (Figure 3E).
Figure 4|Distribution of the endogenous pool of neuroblasts appearing after BNN-20 + graft treatment in the ipsilateral to the graft hemisphere.
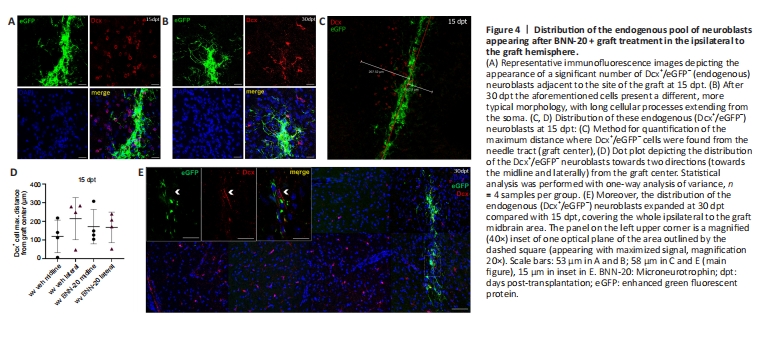
The second, more interesting, observation was the emergence of a population of eGFP–/Dcx+ (Dcx+) cells dispersed around the grafts, indicating the recruitment of endogenous neuroblasts (Figure 4A–C). The emergence of these cells was dependent on the presence of the graft, as evident by their absence both at the contralateral hemisphere (data not shown) and after a sham procedure in which an identical plan of BNN-20/vehicle administration was followed by the injection of suspension medium only (Additional Figure 3A and B). The distribution of endogenous neuroblasts at 15 dpt was restricted at a small area around the graft site, varying from 119.23 ± 43.67 to 214.24 ± 56.39 μm away from the injection needle route (Figure 4C and D). No statistical differences were found between BNN-20 and vehicle-treated mice or between the two directions of migration – towards the midline or laterally (Figure 4C and D). However, the distribution of these neuroblasts was dramatically expanded, away from the graft, at 30 dpt, with Dcx+ cells being detected throughout the whole ipsilateral (left) midbrain area (Figure 4E).