脊髓损伤
-
Figure 2|SCI-induced apoptosis can be alleviated by treatment with Lup.
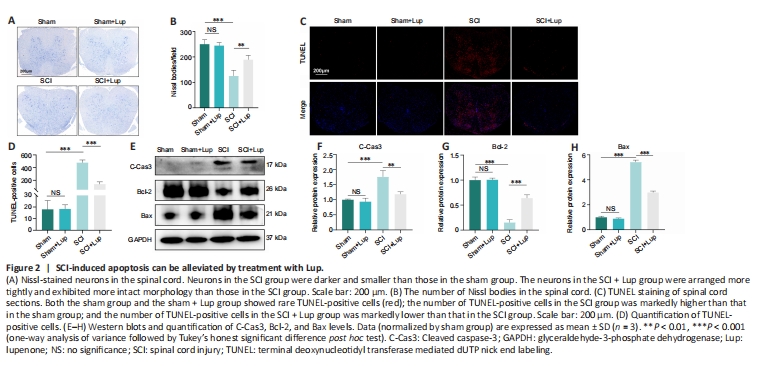
Nissl staining of the spinal cord was carried out to assess the neurons 7 days after SCI. No significant difference was observed between the sham and sham + Lup groups in terms of the number of Nissl bodies (P > 0.05; Figure 2A and B), indicating that Lup had no effect on the spinal cord in the absence of injury. Nissl staining showed that neurons in the SCI group were darker and smaller compared with those in the sham group. There were markedly fewer neurons in the SCI group compared with the sham group. Lup treatment significantly increased the number of neurons (P < 0.01) and restored neuron morphology (Figure 2A and B). Next, a TUNEL assay was performed to assess the number of apoptotic cells 7 days after SCI. There were significantly fewer TUNEL-positive cells in the SCI + Lup group compared with the SCI group (Figure 2C and D). Western blotting showed that expression levels of cleaved caspase-3 and Bax (both key determinants of apoptosis (Gaumer et al., 2000; Abbaszadeh et al., 2020)) were considerably lower in the SCI + Lup group than in the SCI group; in addition, Bcl-2 expression was significantly higher in the SCI + Lup group than in the SCI group (P < 0.001; Figure 2E–H). These results demonstrate that Lup exerted a neuroprotective effect on the injured spinal cord.
Figure 3|Lup improves microglial polarization in SCI mice.
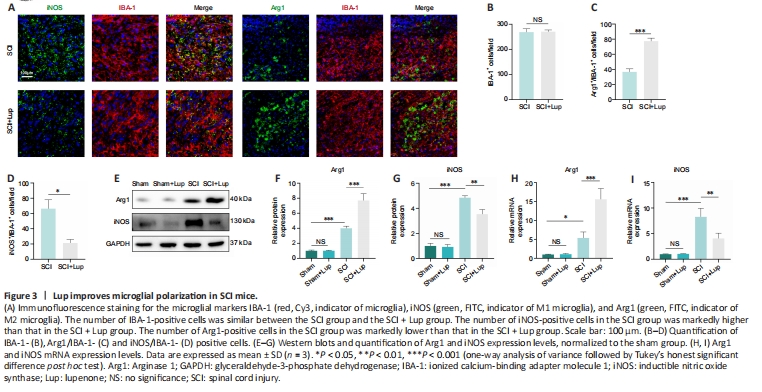
Microglial activation is important for neuroinflammation in SCI (Liu et al., 2020). We investigated whether Lup suppressed microglia-mediated neuroinflammation by regulating microglial polarization. Microglial polarization was evaluated by immunofluorescence staining on 7 days after SCI (Figure 3A). No significant difference was observed in microglia (marked by IBA-1) numbers between the SCI and SCI + Lup groups (P > 0.05; Figure 3A and B). The number of Arg1-positive microglia (M2) was significantly higher in the SCI + Lup group compared with the SCI group (P < 0.001; Figure 3A and C). The number of iNOS-positive microglia (M1) in the SCI + Lup group was significantly lower than in the SCI group (P < 0.05; Figure 3A and D). Western blotting showed that iNOS expression was significantly lower in the SCI + Lup group than in the SCI group (P < 0.01), whereas Arg1 expression in the SCI + Lup group was significantly higher compared with that in the SCI group (P < 0.001; Figure 3E–G). Furthermore, the RT-PCR results showed similar changes (Figure 3H and I). The results demonstrate that Lup promoted a shift in microglial polarization from a proinflammatory phenotype to an anti-inflammatory phenotype.
Figure 5|Lup regulates BV2 cell polarization.
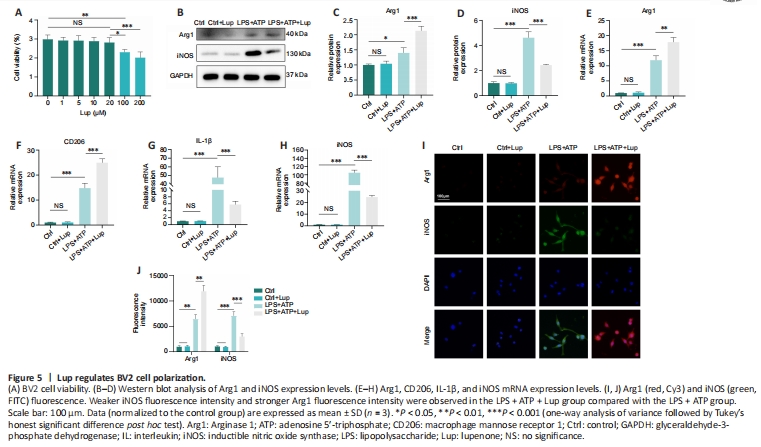
To further explore the effects of Lup on microglia, we performed in vitro experiments in BV2 cells, an alternative to investigating microglia in vitro (Hou et al., 2019). Lup did not cause significant cell death in BV2 cells at concentrations less than or equal to 20 μM (P > 0.05; Figure 5A). Thus, 20 μM of Lup was used to treat BV2 cells in subsequent experiments. In the LPS + ATP-stimulated BV2 cells, Lup significantly reduced expression of the proinflammatory protein iNOS (P < 0.001) and increased expression of the anti-inflammatory protein Arg1 (P < 0.01; Figure 5B–D). Similar changes were observed at the mRNA level: IL-1β and iNOS mRNA levels were markedly lower, and Arg1 and CD206 mRNA levels were higher, in the LPS + ATP + Lup group than in the LPS + ATP group (Figure 5E–H). Next, immunofluorescence analysis was performed to explore the polarization phenotype. Weaker iNOS fluorescence intensity and stronger Arg1 fluorescence intensity were observed in the LPS + ATP + Lup group than in the LPS + ATP group (Figure 5I and J). These results indicate that Lup promoted polarization to the anti-inflammatory M2 phenotype and suppressed polarization to the proinflammatory M1 phenotype in BV2 cells, confirming that Lup treatment improves motor function by altering microglia polarization.
Figure 6|Lup decreases NLRP3 activation and subsequent pyroptosis in BV2 cells.

To verify the role of Lup in inflammasome activation-related inflammation, we detected the expression levels of relevant indicators. NLRP3, GSDMD, and N-GSDMD expression levels increased dramatically in LPS + ATP-stimulated BV2 cells but decreased markedly after Lup treatment (Figure 6A–D). In addition, pro-IL-1β and IL-1β expression levels were markedly lower in the LPS + ATP + Lup group than in the LPS + ATP group (Figure 6A, E, and F). Next, we assessed two indicators of pyroptosis, PI uptake and LDH release (Liu et al., 2020). A significantly greater number of PI-positive cells were observed in LPS + ATP–treated BV2 cells compared with the LPS + ATP + Lup group (P < 0.001; Figure 6G and H). Similarly, as shown in Figure 6I, cells incubated with LPS + ATP released significantly higher levels of LDH (P < 0.001) than cells incubated with LPS + ATP + Lup (P < 0.05; Figure 6I). ELISA assays demonstrated that IL-1β and IL-18 expression levels were markedly lower in the LPS + ATP + Lup group than in the LPS + ATP group (Figure 6J and K). These results indicate that Lup suppressed LPS + ATP-induced NLRP3 activation and alleviated subsequent inflammation in BV2 cells.
Figure 7|CY-09 helps alter microglial polarization and ameliorate pyroptosis in BV2 cells.

To explore the upstream and downstream relationships between NLRP3 and microglia polarization, CY-09 was used to specifically inhibit NLRP3, and microglial polarization was assessed. NLRP3 expression was markedly lower in the LPS + ATP + CY-09 group compared with the LPS + ATP group (Figure 7A and B), indicating that NLRP3 activation was inhibited by CY-09. Next, we assessed expression levels of indicators of microglia polarization, including the anti-inflammatory factor Arg1 and the proinflammatory factor iNOS. Arg1 was expressed at markedly higher levels in the LPS + ATP + CY-09 group than in the LPS + ATP group (Figure 7A and C); and iNOS expression was markedly lower in the LPS + ATP + CY-09 group compared with the LPS + ATP group (Figure 7A and D). These results demonstrate that inhibiting NLRP3 suppresses proinflammatory M1 polarization and enhances anti-inflammatory M2 polarization in BV2 cells. RT-qPCR analysis of NLRP3, Arg1, and iNOS mRNA expression levels yielded similar results (Figure 7E–G). Furthermore, the expression levels of inflammatory cytokines such as IL-1β and IL-18 were markedly lower in the LPS + ATP + CY-09 group compared with those in the LPS+ATP group (Figure 7H and I).
Finally, we confirmed the relationship between NLRP3 and pyroptosis in BV2 cells. The data demonstrated that LDH levels were markedly higher in the LPS + ATP group compared with the LPS+ATP+CY-09 group (Figure 7J). Furthermore, the number of PI-positive cells in the LPS + ATP group was markedly higher than that in the LPS + ATP + CY-09 group (Figure 7K and L). The results demonstrated that inhibiting activation of the NLRP3 inflammasome alters microglial polarization and alleviates pyroptosis in BV2 cells.
Figure 8|Activation status of the NF-κB signaling pathway is regulated by Lup.

The NF-κB pathway can trigger NLRP3 inflammasome activation and regulate microglial polarization (Ye et al., 2019). To determine whether Lup ameliorated inflammation in BV2 cells via the NF-κB pathway, we assessed the interaction between Lup and the NF-κB pathway in the BV2 cells. The molecular docking results (Figure 8A) showed that hydrogen bonds formed between Lup and PHE239, and hydrophobic interactions were observed between Lup and hydrophobic amino acid residues, including LYS28, GLU222, ARG236, and PRO275. In addition, the molecular docking results revealed that the binding energy between Lup and p65 was –8.0 kcal/mol, indicating a stable binding interaction (Figure 8A). We then assessed the activation status of the NF-κB signaling pathway in BV2 cells treated with LPS + ATP or LPS + ATP + Lup. Whole-cell western blotting demonstrated that Lup did not affect p65, p-p65, IκBα, and p-IκBα expression levels in the absence of LPS + ATP (Figure 8B–G). However, p-p65 and p-IκBα expression levels in the LPS + ATP group were markedly higher than those in the control group (Figure 8B–G), and the levels of both p-p65 and p-IκBα in the LPS + ATP + Lup group were markedly lower than those in the LPS + ATP group (Figure 8B–D). Furthermore, p-p65 expression in the nucleus was markedly lower in the LPS + ATP + Lup group than in the LPS + ATP group; and p65 expression in the cytoplasm was markedly higher in the LPS + ATP + Lup group than in the LPS + ATP group (Figure 8E–G). Therefore, Lup may suppress over-activation of the NF-κB pathway. To confirm this, we overexpressed p65, the key protein in the NF-κB pathway (Jiang et al., 2013; Liu and Su, 2023) in BV2 cells and validated effective overexpression by detecting p65 mRNA levels (Figure 8H). Both p65 and p-p65 levels were substantially higher in the LPS + ATP + OE group than in the LPS + ATP + vector group (Figure 8I–K). In addition, p-p65 expression was substantially lower in the LPS + ATP + OE + Lup group than in the LPS + ATP + OE group (Figure 8I and J), indicating that p65 activation was inhibited by Lup. Nuclear translocation of the p65 subunit is regarded as an indicator of NF-κB activation (Zusso et al., 2019). Immunofluorescence analysis showed that Lup treatment substantially reduced the increase in p65 translocation caused by LPS + ATP stimulation (Figure 8L and M). Furthermore, p65 overexpression further increased p65 translocation. However, the increased p65 translocation caused by p65 overexpression was markedly reversed by Lup treatment (Figure 8N and O). These findings suggest that Lup inhibits overactivation of the NF-κB pathway by inhibiting the function of the p65 subunit.
Figure 9|Lup ameliorates overactivation of the NLRP3 inflammasome and NLRP3 activation-induced microglial polarization and pyroptosis through the NF-κB pathway in BV2 cells.
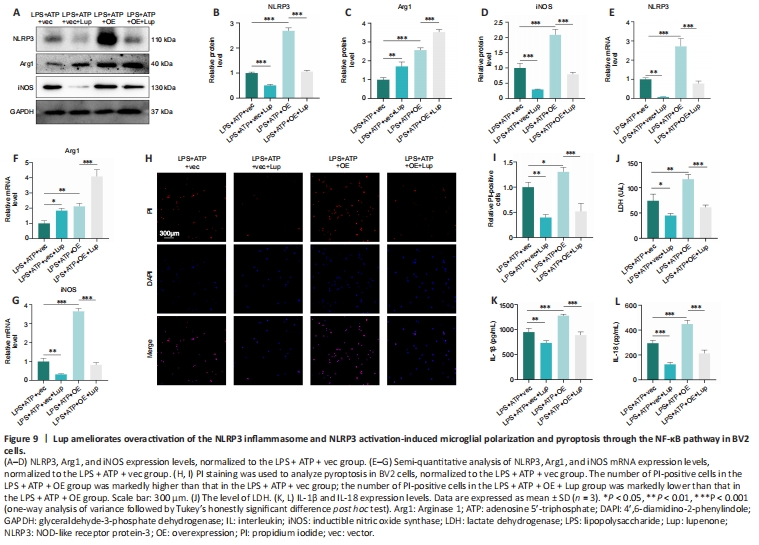
Next, we evaluated the effects of Lup on NLRP3 activation, microglial polarization, and pyroptosis in the BV2 cells overexpressing p65. NLRP3 expression was markedly higher in the LPS + ATP + OE group compared with the LPS + ATP + vector group and the LPS + ATP + OE + Lup group (Figure 9A and B), indicating that the NLRP3 inflammasome is regulated by p65. Then, microglial polarization was assessed by western blot, which showed that p65 overexpression increased the levels of both Arg1 (a marker of anti-inflammatory M2 polarization) and iNOS (a marker of proinflammatory M1 polarization; Xie et al., 2021; Li et al., 2023) (Figure 9A, C, and D). Treatment with Lup intervention further increased expression of the anti-inflammatory factor Arg1 and decreased expression of the proinflammatory factor iNOS (Figure 9A, C, and D). RT-PCR analysis yielded similar results (Figure 9E–G). These results demonstrate that Lup inhibited M1 polarization and promoted M2 polarization by suppressing NF-κB pathway-mediated activation of the NLRP3 inflammasome.
Next, we assessed the expression of pyroptosis indicators. The number of PI-positive cells increased in cells overexpressing p65, and this effect was markedly reversed by Lup treatment (Figure 9H and I). Similarly, the elevation in LDH levels caused by p65 overexpression was markedly reversed by treatment with Lup (Figure 9J). Furthermore, the expression levels of inflammatory cytokines such as IL-1β and IL-18 were increased in the LPS + ATP + OE group and markedly suppressed by Lup (Figure 9K and L). Taken together, these findings suggest that Lup ameliorates overactivation of the NLRP3 inflammasome and NLRP3 activation-induced microglia polarization and pyroptosis by inhibiting the NF-κB pathway.