视神经损伤
-
Figure 4|LbGP improves photoreceptor structure and survival in the MNU-injured retina.
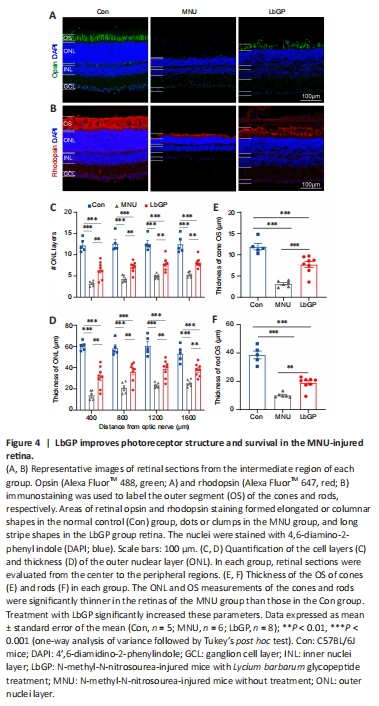
Responses assessed by ERG were improved in LbGP-treated mice, suggesting that LbGP slowed the MNU-induced cellular degeneration of photoreceptors. Therefore, we examined the survival and structures of photoreceptors in these mice by immunostaining. Because the effects of MNU may vary from the center to the periphery of the retina (Tao et al., 2015), we examined the morphological changes induced by MNU at different regions of the retina. Figure 4A and B shows typical images of a retinal section from the intermediate region in each group. Compared with the Con group, the ONL (where the photoreceptor soma are located) was much thinner, with fewer cell layers, in the MNU group, but showed improvements in terms of thickness and number of cell layers in the LbGP group. At 800 μm from either side of the optic nerve center, the number of cell layers and the thickness of the ONL were significantly reduced in the MNU group compared to those in the Con group (P = 0.0047) and were improved in the LbGP group (P = 0.0038; Figure 4C and D). The 800-μm location showed the greatest effects of LbGP: ONL thickness, which was reduced by 63.9% in the MNU group compared to that in the Con group, was increased by 72.1% in the LbGP group (n = 8, P < 0.01 compared to the MNU group; Figure 4D), while the number of cell layers in the ONL increased by 65.5% in the LbGP group (n = 8, P < 0.01 compared to the MNU group; Figure 4C).
We conducted further examinations of the cone and rod structures by immunostaining the OSs using antibodies against opsin and rhodopsin, respectively (Carroll et al., 2012). While the OS of the cones in the Con group presented as long strips, those in the MNU group were barely stained, with only a few dots; however, LbGP restored the morphology of long strips (Figure 4A). Similarly, the OS of rods showed a clear, thick layer of immunostaining in the Con group that was greatly reduced in the MNU group and restored in the LbGP group (Figure 4B). In the intermediate region of the retina (800 μm), compared with the Con group, measurements of OS thickness of cones and rods were decreased by 73.5% and 73.7%, respectively, in the MNU group (n = 8, P < 0.01). By contrast, compared with the MNU group, the LbGP group showed increases in cone and rod OS thickness of 143.6% and 86.03%, respectively (n = 8, P < 0.01; Figure 4E and F).
Figure 5|LbGP partially inhibits microglial activation in the MNU-injured retina.
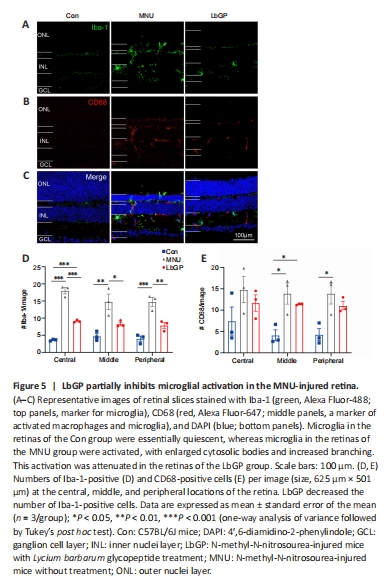
We also investigated possible mechanisms for the protective effect of LbGP on degeneration of the retina. Reactive gliosis, a hallmark of retinal inflammation, is characterized by the activation of microglia and Müller cells (Bringmann et al., 2009). Because LbGP has an anti-inflammatory effect (Huang et al., 2022), we examined whether LbGP inhibited reactive gliosis. The activation of microglia was examined by immunostaining for the activated microglial markers Iba-1 (markers of microglia) (Kotliarova and Sidorova, 2021) and CD68 (markers of active microglia) (Slepko and Levi, 1996). In the normal retinas of Con group mice, there was negligible expression of Iba-1 and CD68, indicating minimal microglial activation and phagocytic activity. MNU group mice showed significantly increased retinal expression of CD68 and Iba-1, indicating increased microglial activation, and a considerable number of microglia with reactive morphology characterized by an amoeboid shape. LbGP treatment reduced the expression of both Iba-1 and CD68 (Figure 5A–C). In all retinal regions from the center to the periphery, compared to the MNU group, the LbGP group showed significantly reduced numbers of Iba-1-positive cells (n = 3, P < 0.05; Figure 5D) and a tendency for reduced CD68-positive cells (n = 3, P > 0.05, compared to MNU group, Figure 5E). Therefore, we concluded that LbGP inhibited the reactivation of microglia in the MNU-injured retina.
Figure 6|LbGP hardly inhibits Müller glial cell activation in the MNU-injured retina.
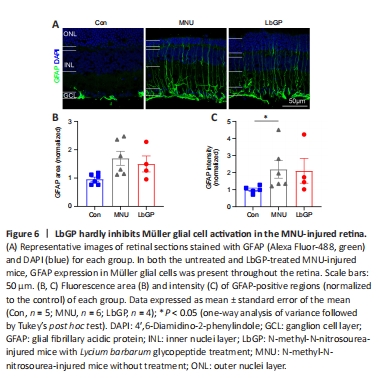
Next, we observed the status of Müller glial cells, which play a crucial role in maintaining retinal homeostasis (Bringmann et al., 2009), in each group. The activation of Müller cells is indicated by increased GFAP expression in the cell processes (Bringmann et al., 2006). In Con group retinas, Müller glial cells did not exhibit any signs of activation, and GFAP expression was mainly restricted to the astrocytes in the ganglion cell layer and was accompanied by short dendritic extensions. In MNU group retinas, there was a marked increase in GFAP expression in the processes of Müller glial cells. These processes exhibited strong GFAP immunoreactivity and extended from the ganglion cell layer to the ONL, indicating a pronounced activation of Müller glial cells. The LbGP treatment group showed only a slight decrease in retinal GFAP expression (n = 4, P > 0.05 compared to the MNU group; Figure 6). We concluded that, in the MNU-injured retina, LbGP partially inhibited microglial activation but did not affect Müller cells.