神经退行性病
-
Figure 3|Effects of ICA on the pathological injury in the cerebral cortex of 3×Tg-AD mice.
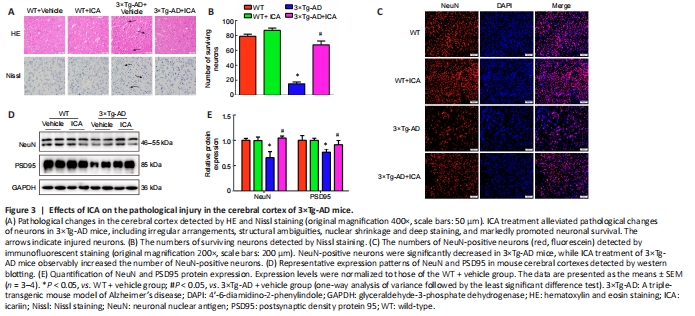
To investigate whether ICA alleviated histopathological changes in 3×Tg-AD mice, we performed HE and Nissl staining to observe the pathological changes and number of neurons in the cerebral cortex. HE staining showed that cerebral neurons in the WT mice exhibited regular arrangement with distinct edges and clear nuclei and nucleoli (Figure 3A). However, in the 3×Tg-AD + vehicle group, cerebral neurons displayed an irregular arrangement, structural ambiguity, nuclear shrinkage, and deep staining, which was significantly ameliorated by ICA treatment (Figure 3A). Additionally, Nissl staining demonstrated a significant loss of cerebral neurons in the 3×Tg-AD + vehicle group compared with that in WT mice. ICA treatment markedly promoted the survival of cerebral neurons compared with that in the vehicle-treated 3×Tg-AD mice (Figure 3A and B).
To further confirm the neuroprotective effect of ICA, the number of NeuN-positive cells and NeuN protein expression levels were used to evaluate the effects of ICA on neurons in the cerebral cortex. NeuN is a neuronal marker for mature neurons (Mullen et al., 1992). As shown in Figure 3C–E, ICA treatment observably increased the number of NeuN-positive neurons and NeuN protein levels compared with those in the 3×Tg-AD + vehicle group.
Synapse loss is a pathological basis of memory dysfunction (Asok et al., 2019), and PSD95, which is a synaptic marker (Coley and Gao, 2018), was used to evaluate the effects of ICA on synapse integrity. As expected, ICA treatment of 3×Tg-AD mice observably increased PSD95 expression compared with that in the 3×Tg-AD + vehicle group (Figure 3D and E). These results showed that ICA treatment protected neurons and synapses from damage in 3×Tg-AD mice.
Figure 4|Effects of ICA on AD-like pathology in the cerebral cortex of 3×Tg-AD mice.

To explore whether the neuroprotective effect of ICA in 3×Tg-AD mice was achieved by the amelioration of AD markers, Aβ accumulation and tau hyperphosphorylation in the cerebral cortex were evaluated. Treatment with ICA markedly attenuated Aβ accumulation in the cerebral cortexes of 3×Tg-AD + ICA mice compared with that in the 3×Tg-AD + vehicle group (Figure 4A). To confirm these results, we performed western blot analysis to assess ICA-mediated modulation of Aβ deposits. A significant decrease in levels of the Aβ peptide precursor APP and Aβ1–40 and Aβ1–42 peptides, both of which are the main components of senile plaques (SPs) (Seino et al., 2021), was observed in the 3×Tg-AD + ICA group compared with those in the 3×Tg-AD + vehicle group (Figure 4B and C). The accumulation of hyperphosphorylated tau in AD brain is another important pathological hallmark, and more than 40 hyperphosphorylated sites have been documented (Kimura et al., 2018), including Thr217, Ser199/202, Thr231, and Ser396/404. In the present study, ICA treatment markedly decreased hyperphosphorylated tau at the Thr217, Ser199/202, and Thr231 sites when compared with that in the 3×Tg-AD + vehicle group (Figure 4D and E). These results suggested that ICA alleviated AD-like pathology in 3×Tg-AD mice.