中国神经再生研究(英文版) ›› 2024, Vol. 19 ›› Issue (4): 872-880.doi: 10.4103/1673-5374.382255
长非编码RNA H19调节闭合性颅脑损伤模型小鼠诱导神经干细胞的神经发生
Long non-coding RNA H19 regulates neurogenesis of induced neural stem cells in a mouse model of closed head injury
Mou Gao1, 2, 3, *, Qin Dong4, Zhijun Yang3, Dan Zou1, Yajuan Han3, Zhanfeng Chen3, *, Ruxiang Xu1, *
- 1Department of Neurosurgery, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan Province, China; 2Department of Neurosurgery, Chinese PLA General Hospital, Beijing, China; 3Zhongsai Stem Cell Genetic Engineering Co., Ltd., Sanmenxia, Henan Province, China; 4Department of Neurology, Fu Xing Hospital, Capital Medical University, Beijing, China
摘要:
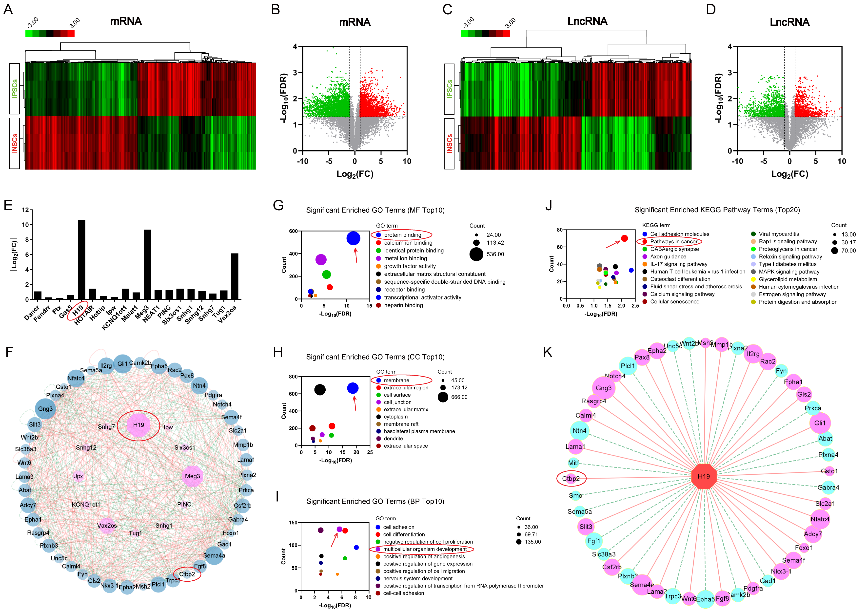
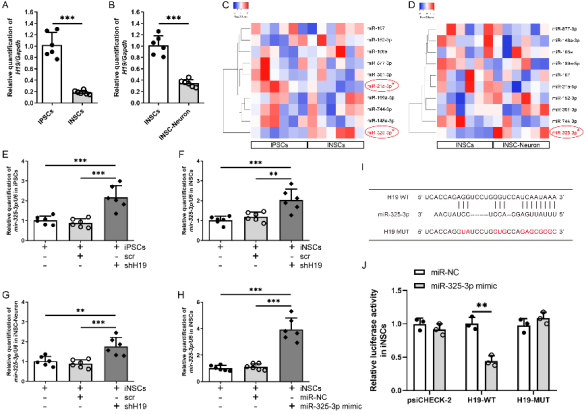
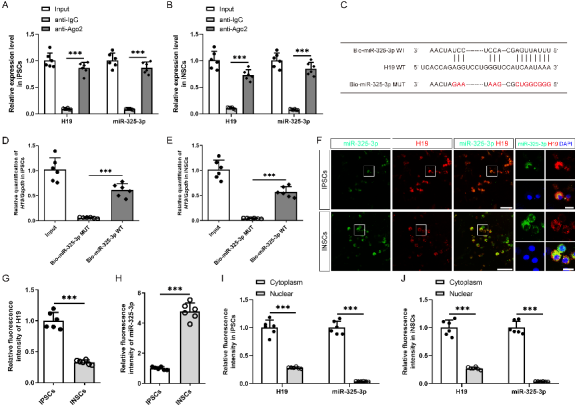
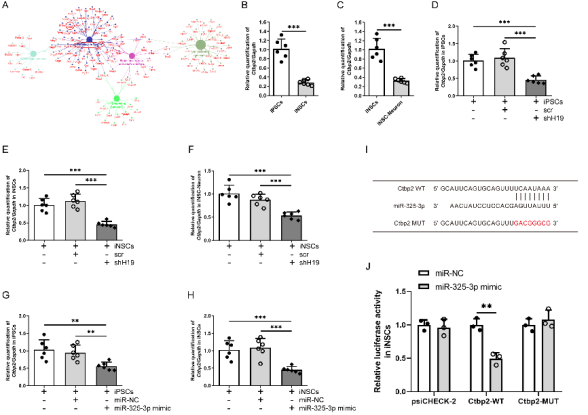
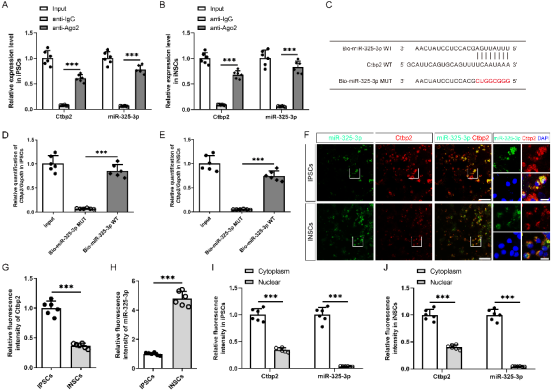
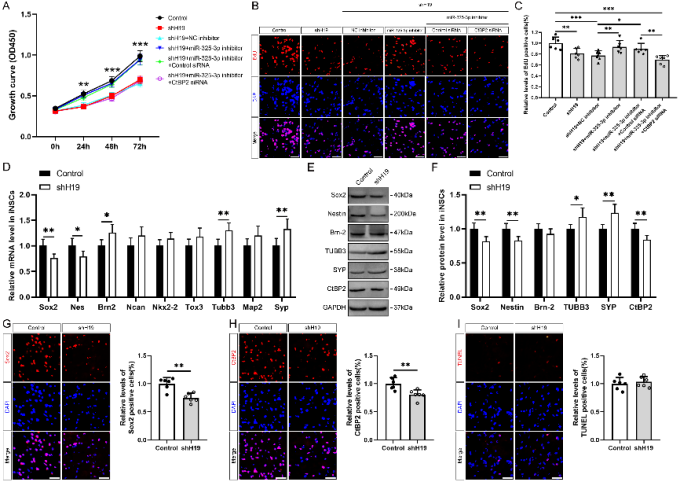
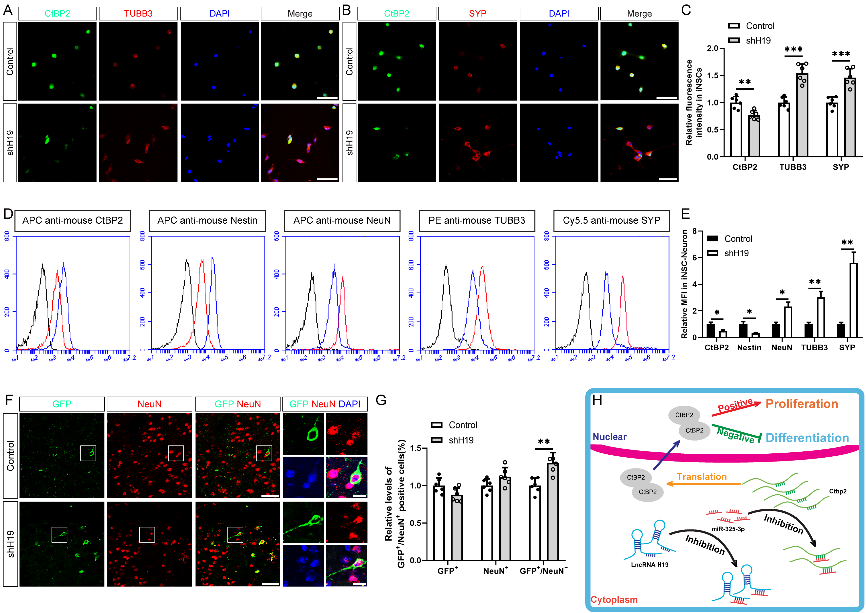
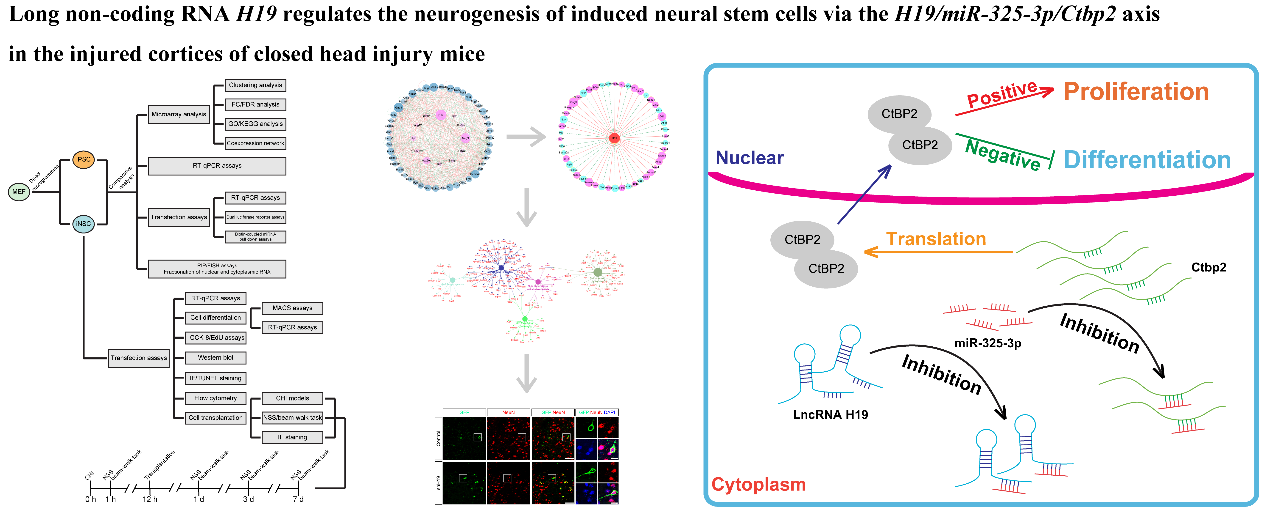
作者曾报道诱导神经干细胞具有类似于神经干细胞的自我更新和定向分化为成熟神经元的潜力,然而脑损伤后移植诱导神经干细胞分化为成熟神经元以替代受损神经元的作用仍较为有限,此外,调控诱导神经干细胞神经发生的机制也不明确。实验对比分析了由体细胞经过体外重编程操作直接获取的诱导多能干细胞与诱导神经干细胞的全基因表达谱芯片数据,筛选出与神经发生相关的重要调控分子,随后进行功能学、双荧光素酶报告基因、RNA免疫沉淀、生物素偶联miRNA沉降、荧光原位杂交和干细胞移植等实验,以阐明诱导神经干细胞的神经发生机制,并进一步评估其对于脑损伤的治疗潜力。结果发现,与诱导多能干细胞相比,H19是诱导神经干细胞中下调最多的神经发生相关的长非编码RNA。H19在诱导神经干细胞中的水平明显低于诱导多能干细胞,但大大高于诱导神经干细胞来源的神经元。然后,实验预测了H19的靶基因,发现H19直接与miR 325 3p相互作用,而后者在诱导多能干细胞和诱导神经干细胞中可以直接与Ctbp2相互作用。沉默H19或Ctbp2损害了诱导神经干细胞的增殖,而miR-325-3p的抑制恢复了H19抑制的效果,但没有恢复Ctbp2抑制的效果。H19沉默未能诱发诱导神经干细胞的明显损伤,但却明显促进了诱导神经干细胞的神经分化。值得注意的是,在诱导神经干细胞移植中沉默H19明显促进了闭合性颅脑损伤小鼠的神经系统恢复。以上结果表明,H19可通过H19/miR 325 3p/Ctbp2轴调控诱导神经干细胞的神经发生。值得注意的是,沉默H19可通过H19/miR 325 3p/Ctbp2轴促进诱导神经干细胞分化为神经元。因而,H19/miR 325 3p/Ctbp2轴调控诱导神经干细胞神经发生有潜力用于创伤性脑损伤的治疗。
https://orcid.org/0000-0002-1300-5535 (Ruxiang Xu); https://orcid.org/0009-0009-3254-5535 (Zhanfeng Chen); https://orcid.org/0009-0000-1058-1169 (Mou Gao)