NRR:解放军总医院潘隆盛和宗睿团队报道中国首批特发性震颤磁波刀临床试验1年结果
撰写:宗睿,李雪梅,尹春宇,娄昕,余新光,潘隆盛,等
近期,解放军总医院第一医学中心潘隆盛团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)2024年第9期上发表了研究论文,对中国首台“磁波刀”治疗特发性震颤临床试验,所有受试者都接受了靶向丘脑腹侧中间核的治疗。1年随访结果显示,临床震颤评分量表(CRST)总分基线的估计边际平均值为58.3±3.6,治疗后12个月改善到23.1±6.4。共记录了50个不良事件。该文带来的启示:“磁波刀”对于药物难治性特发性震颤的治疗,在中国人群也同样取得较为满意的临床结果,治疗安全性好,相关治疗技术值得推广。宗睿、李雪梅和尹春宇为论文共同第一作者,娄昕、余新光和潘隆盛教授为论文通讯作者。
特发性震颤(ET)是最常见的运动障碍之一,典型的特征是频率为8-12赫兹的姿势性和意向性震颤,通常累及上肢。ET可以使用药物进行治疗,然而大约50%的患者由于无效或不耐受而最终停止用药。手术干预包括,丘脑腹中间核(Vim)射频(RF)消融,立体定向放射外科(SRS)或脑深部电刺激(DBS)。但许多患者不愿意接受侵入性手术或电离辐射治疗。磁共振引导经颅超声聚焦治疗(MRgFUS)是一种新开发的治疗技术[1]。美国、欧洲、日本、韩国、以色列以及其他国家和地区的监管机构已经批准使用经颅磁共振治疗ET[2]。MRgFUS靶向Vim治疗帕金森病震颤同样获得较好的效果[3, 4]。
2018年8月,解放军总医院安装了中国第一台MRgFUS系统。设备安装后,该中心随即启动了中国地区首批关于ET治疗的临床试验研究。这项临床试验的目的是验证MRgFUS在中国人群中治疗药物难治性ET的安全性和有效性。
研究方法:该研究是全球前瞻性多中心临床试验(ClinicalTrials.gov Identifier: NCT03253991)的一部分,由InSightec有限公司赞助。作为多中心临床试验的一部分,解放军总医院试验中心分配了10例样本进行治疗。 这项前瞻性、单中心、单臂研究得到了解放军总医院伦理委员会的批准(2018-021,11/10/2018)。 主要的纳入排除标准包括:年龄在22岁或以上;由专门运动障碍神经科医生确定诊断;对药物治疗反应不足;临床震颤评价量表(CRST)[5]姿势性或意向性震颤严重程度得分大于或等于2分,残疾(在CRST C部分的16-23项中任何一项得分2或以上)。排出标准包括:不包括有严重系统性疾病的受试者;有异常出血和/或凝血病史,或有出血的危险因素;有MR成像的禁忌症;在治疗过程中不能或不愿意忍受所要求的长期静止的仰卧姿势; 存在任何其他神经退行性疾病、明显的认知障碍或精神病史;脑血管疾病、脑肿瘤、癫痫发作或其他脑部疾病;接受过深部脑刺激或先前立体定向消融的受试者;整体颅骨密度比(SDR)为0.3(±0.05)或更低。治疗靶点定位方法:Vim核坐标位于前汇合点-后汇合点(AC-PC)平面,PC点前方25%处的外侧14mm,如果第三脑室较大,则为第三脑室壁外侧11.5mm。
主要终点是评估治疗的安全性和有效性。安全性将通过评估设备/治疗相关并发症的发生率和严重程度来确定。不良事件将由研究人员报告并进行分类。疗效使用CRST量表进行评价。研究的次要终点是评估受试者的日常功能:通过CRST Part-C测量。临床评估时间包括:基线以及治疗后1个月、3个月、6个月、12个月。对于重复测量资料,使用混合线性模型进行分析。
结果分析:有10名受试者入选,其中7名男性和3名女性。平均年龄为65.2±5.2岁,平均病程为18.4±9.9年。CRST总分结果显示所有受试者治疗后震颤立即得到改善,其中7名受试者在1年时治疗结果得到保持,另外3名受试者震颤部分复发。这3名复发的受试者基线评分明显高于其他7名受试者。所有7名治疗侧震颤控制良好的患者的CRST C部分得分几乎为0,意味着他们生活质量几乎恢复正常。CRST总分的估计边际平均值从基线58.3±3.6(50.4-66.2)提高到12个月随访的23.1±6.4(8.9-37.3),手部得分从基线22. 8±1.4(16.9-26.0)到12个月的随访6.5±2.5(0.9-12.1),Part-C得分从基线16.0±1.0(13.7-18.3)到12个月的随访3.9±1.8(-0.0-7.8)。
安全性评价方面,记录了50个不良事件(AE),其中2个被定义为严重不良事件(SAE)。 发生的最常见的术中AE是恶心或呕吐(6例患者)和头痛(3例患者)。这些AE通常发生在治疗中给予能量过程,一旦超声处理结束,症状就会很快得到缓解。最常见的术后AE是舌头或脸颊麻木(6例患者),头皮麻木(4例患者),头皮痛觉减退(2例患者),以及平衡障碍(4例患者)。在12个月的随访中,4例患者的舌头或脸颊麻木明显缓解,另外2例患者的麻木完全解决。在4例有平衡问题的患者中,3例患者的副作用在几天内完全解决,第4例患者的不平衡问题持续了5个月,需要康复训练才能完全恢复。其他报告的AE包括,肢体痉挛,由于膜对头皮的压力造成的压迫性疼痛,声音嘶哑,过度流涎等。所有这些AE都是轻度的。
发生的两个SAE。一名患者出现了短暂的右上肢无力,这是由于内囊水肿引起的(肌力IV+)。8天后肢体力量恢复正常。第二个SAE与治疗无关,一名患者在治疗9个月后报告了新发的心绞痛并伴有心肌梗死,该患者接受了顺利的冠状动脉支架手术,症状得到缓解。
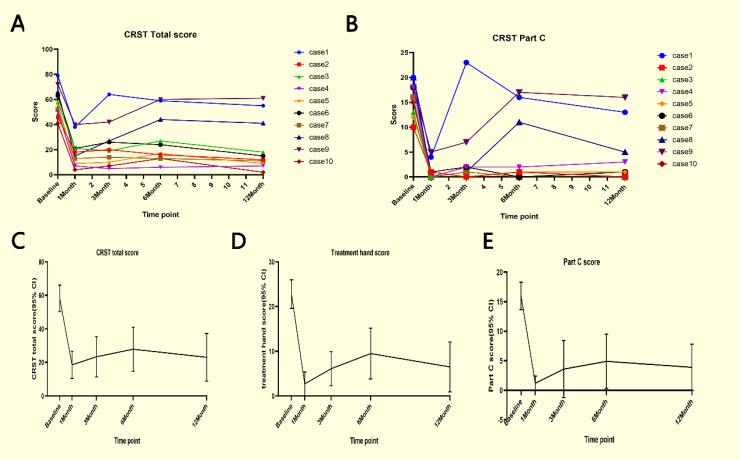
图1. 治疗前后10名受试者的CRST评分。(A和B)每个受试者治疗前基线及随访时的CRST总分和C部分得分。7名受试者有明显改善,3名受试者(病例1,8,9)震颤部分复发。(C、D和E)总结CRST总分,以及各组受试者的手部得分和C部分得分。线形图代表估计的边际平均值和平均值的95%置信区间。
该治疗中心的首批“磁波刀”治疗ET临床结果,与国外临床报告的结果基本一致。一项包含76名的随机对照研究中,患者按3:1比例被随机分配到磁波刀治疗组和假治疗组。术后3个月,治疗组的手震颤平均得分改善了47%[6]。Jung等人在一项针对20名药物难治性ET患者的单中心试验中,报告了MRgFUS丘脑切开术后一年的平均CRST总分改善率为67.3%[7]。一项对154名接受磁波刀治疗的ET患者进行的荟萃分析(Mohammed等,2018)发现,治疗后CRST总分、CRST A部分和CRST C部分得分分别提高了62.2%、62.4%和69.1%。从生活质量上看,即使是单侧治疗,治疗侧手的震颤控制良好,可持续改善生活质量大于50%[8, 9]。在该研究的队列中,CRST C部分的平均得分改善率为75.6%。这代表患者的日常活动和社会生活有意义的功能改善。关于治疗安全性,本组报告不良事件较多,与临床方案要求严格,报告更加积极有关。其中最常见的不良事件是术中的恶心、头痛,与能量传递有关,能量传递停止后症状均能立即缓解。术后最常见的不良事件是麻木,包括舌尖、头皮等部位麻木,其次是平衡障碍。这些副作用可能是由于毁损灶周围水肿扩散到到感觉通路内侧丘系有关。这些症状都比较轻微,不会对生活质量造成明显的损害,大多数常在3个月内消退。报告的2例严重不良事件,一件是术后患者出现治疗侧肌力下降,可能是毁损灶水肿累及内囊,延长了住院,但经过治疗很快缓解。另一件是与治疗无关的,在随访期间新发心肌梗死,患者接受了介入治疗后病情稳定。总之本中心这组临床治疗表明,“磁波刀”治疗ET在中国人群也表现出良好的效果,治疗组很快掌握治疗技术,临床效果可比国外其他中心的治疗质量。MRgFUS可以作为治疗药物难治性ET的一种选择。
原文链接:https://doi.org/10.4103/1673-5374.391192
参考文献
[1] Wang TR, Bond AE, Dallapiazza RF, et al. Transcranial magnetic resonance imaging-guided focused ultrasound thalamotomy for tremor: technical note. Neurosurg Focus. 2018;44(2):E3.
[2] Fishman PS. Thalamotomy for essential tremor: FDA approval brings brain treatment with FUS to the clinic. J Ther Ultrasound. 2017;5:19.
[3] Zaaroor M, Sinai A, Goldsher D, et al. Magnetic resonance-guided focused ultrasound thalamotomy for tremor: a report of 30 Parkinson's disease and essential tremor cases. J Neurosurg. 2018;128:202-210.
[4] Yin C, Zong R, Song G, et al. Comparison of motor scores between OFF and ON states in tremor-dominant Parkinson's disease after MRgFUS treatment. J Clin Med. 11:4502.
[5] Stacy MA, Elble RJ, Ondo WG, et al. Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Mov Disord. 2007;22:833-838.
[6] Elias WJ, Lipsman N, Ondo WG, et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med. 2016;375:730-739.
[7] Jung NY, Park CK, Chang WS, et al. Effects on cognition and quality of life with unilateral magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Neurosurg Focus. 2018;44:E8.
[8] Halpern CH, Santini V, Lipsman N, et al. Three-year follow-up of prospective trial of focused ultrasound thalamotomy for essential tremor. Neurology. 2019;93:e2284-2293.
[9] Sinai A, Nassar M, Eran A, et al. Magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: a 5-year single-center experience. J Neurosurg. 2019:1-8.
潘隆盛:解放军总医院神经外科医学部功能神经外科主任医师、副教授、硕士生导师。擅长帕金森病、特发性震颤、肌张力障碍、癫痫、脑瘫、痉挛性斜颈、舌咽神经痛微血管减压等功能神经外科治疗;开展头颈部肿瘤和血管畸形及脊柱脊髓疾病的射波刀、ZAP-X放射外科治疗,国内第一个开展高能超声聚焦系统“磁波刀”治疗特发性震颤和帕金森震颤。担任中国研究型医院学会肿瘤放射生物与多模态诊疗专业委员会副主任委员、世界华人神经外科协会放射神经外科专业委员会常委。获国家科技进步二等奖1项,军队科技进步一等奖1项、二等奖2项。副主编专著2部。
该项目得到国家老年疾病临床医学研究中心开放课题资助,课题名称:磁共振引导经颅超声聚焦系统(磁波刀)治疗老年特发性震颤的规范化研究 (NCRCG-PLAGH-2019005)。
潘隆盛教授在给患者治疗的场景,左一为潘隆盛教授,患者治疗中震颤即得到缓解,可以稳定的端住水杯。

潘隆盛教授和余新光教授在磁共振的操作台,操作治疗参数。左2为余新光教授,左三为潘隆盛教授。


