中国神经再生研究(英文版) ›› 2025, Vol. 20 ›› Issue (5): 1309-1323.doi: 10.4103/NRR.NRR-D-23-01566
贯穿在阿尔茨海默病发生发展中的microRNA-146a
The complex effects of miR-146a in the pathogenesis of Alzheimer’s disease
Yunfan Long1 , Jiajia Liu2, 3 , Yu Wang4, * , Haidong Guo2, 3, * , Guohong Cui 1, *
- 1 Department of Neurology, Shanghai No. 9 People’s Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China; 2 Academy of Integrattve Medicine, Shanghai University of Tradittonal Chinese Medicine, Shanghai, China; 3 School of Integrattve Medicine, Shanghai University of Tradittonal Chinese Medicine, Shanghai, China; 4 Department of Neurology, Shuguang Hospital Afffliated to Shanghai University of Tradittonal Chinese Medicine, Shanghai, China
摘要:
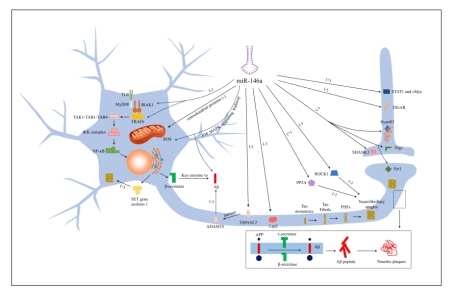
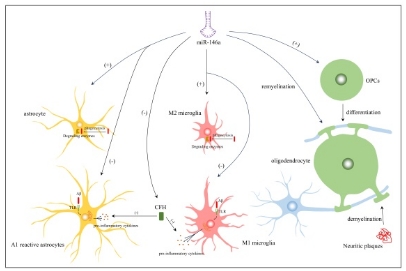
阿尔茨海默病是一种以认知功能障碍和行为异常为特征的神经退行性疾病。淀粉样β蛋白在细胞外沉积形成的神经炎症斑块和高磷酸化tau蛋白在细胞内沉积形成的神经纤维缠结构成了阿尔茨海默病的两个典型病理特征。除了对症治疗外,目前还没有有效的疗法来延缓阿尔茨海默病的病情发展。微小RNA(miRNA)是一种非编码小RNA,可在转录和翻译水平上负向调控基因表达,在多种生理和病理过程中发挥重要作用。miR-146a是NF-κB相关基因的转录因子,通过各种途径广泛参与了阿尔茨海默病的发病。研究表明miR-146a在阿尔茨海默病早期就出现显著失调,并贯穿阿尔茨海默病的整个病理过程。但目前miR-146a在阿尔茨海默病发生发展中起促进作用还是抑制作用仍有较大争议,相关研究也表现出互相矛盾的结果。此综述阐述了miR-146a通过靶向多个mRNA从而对淀粉样β蛋白沉积、tau蛋白过度磷酸化、突触和线粒体功能障碍、神经元死亡、神经胶质细胞功能障碍等阿尔茨海默病的诸多病理特征造成深远影响。同时,还回顾了近年来miR-146a作为临床诊断标志物和治疗药物的相关研究,肯定了其在临床应用方面的广阔前景。
https://orcid.org/0009-0005-7281-3734 (Yu Wang); https://orcid.org/0000-0003-1641-9562 (Haidong Guo); https://orcid.org/0009-0002-2736-9847 (Guohong Cui)
 #br#
#br#
 #br#
#br#