中国神经再生研究(英文版) ›› 2025, Vol. 20 ›› Issue (9): 2682-2696.doi: 10.4103/NRR.NRR-D-23-01539
精氨酸甲基转移酶6调控hnRNP F表达成为调控神经病理性疼痛的潜在干预靶点
Protein arginine methyltransferase-6 regulates heterogeneous nuclear ribonucleoprotein-F expression and is a potential target for the treatment of neuropathic pain
Xiaoyu Zhang1, 2, 3, #, Yuqi Liu2, #, Fangxia Xu2, #, Chengcheng Zhou2 , Kaimei Lu2 , Bin Fang2 , Lijuan Wang2, *, Lina Huang2, *, Zifeng Xu1, 3, *
- 1 Department of Anesthesiology, International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China; 2 Department of Anesthesiology, Shanghai General Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China; 3 Shanghai Key Laboratory of Embryo Original Diseases, Shanghai, China
摘要:
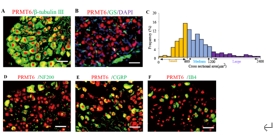
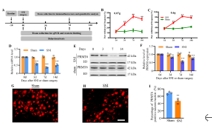
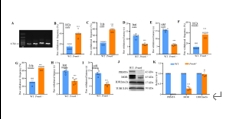
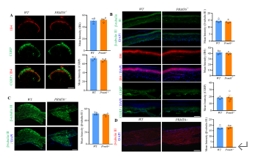
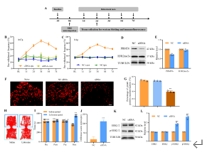
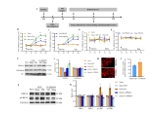
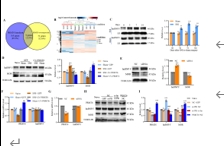
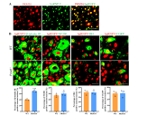
精氨酸甲基转移酶6(PRMT6)功能包括参与RNA代谢、转录调节、细胞内信号转导、DNA修复等,对于真核基因表达调控发挥着重要的作用,但其在背根神经节内的表达以及在神经病理性疼痛中的作用仍未见报道。实验使用坐骨神经分支选择损伤模型研究了 PRMT6 在神经病理性疼痛中的表达和机制。免疫组化、Western 印迹、免疫沉淀和非标记蛋白质组学分析结果显示,PRMT6主要与背根神经节中的β-微管蛋白III共定位,并在坐骨神经分支选择损伤后减少,Prmt6-/-小鼠表现出痛觉过敏。此外,通过抑制PRMT6的减少,可减轻坐骨神经分支选择损伤引起的机械痛超敏反应。相反,背根神经节中 PRMT6 的下调会导致同侧背角中磷酸化的细胞外信号调节激酶水平升高,并在没有坐骨神经分支选择损伤的情况下增强对机械刺激的反应。此外,PRMT6调节hnRNP F的表达并不依赖于其精氨酸甲基化酶活性,而依赖于其319-388个氨基酸结构域。这些结果表明,PRMT6 是治疗周围神经病理性疼痛的潜在干预靶点。
https://orcid.org/0000-0003-4899-4991 (Zifeng Xu); https://orcid.org/0000-0002-4312-2628 (Lina Huang);
https://orcid.org/0000-0002-5359-2084 (Lijuan Wang)