脊髓损伤
-
Figure 1|Timeline of the experimental procedures.

In the present study, 111 out of 126 rats completed the experiment and were analyzed. Nine animals were excluded for at least one of the following reasons: impaired motor function, displacement of electrodes, undetectable motor threshold because of damage to the electrodes, significant reduction of body weight, or deteriorating health conditions. Another six rats did not develop mechanical hypersensitivity (> 50% reduction of MWT from the pre-injury baseline) on day 2 post-SNL; these rats were excluded from the subsequent studies. The general experimental procedure is shown in Figure 1.
Figure 2|Effects of HF-SCS on the ipsilateral mechanical withdrawal thresholds and expression of autophagy markers in the ipsilateral spinal cord in neuropathic pain rats.
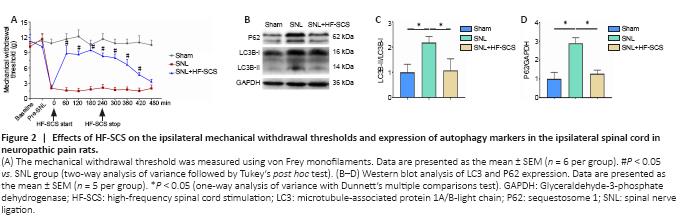
The results from Experiment 1 demonstrated that electrode implantation did not significantly affect the MWTs of rats in any groups compared with their pre-implantation values (Figure 2A, pre-SNL vs. baseline, respectively). Tactile hypersensitivity was observed in the SNL and SNL + HF-SCS groups at the pre-HF-SCS time point (both P < 0.05, vs. pre-SNL, respectively), which was 2 days after SNL surgery. This finding suggests that tactile hypersensitivity was successfully induced by SNL, as has been described in previous studies (Kim and Chung, 1992). HF-SCS treatment significantly increased MWTs to maximal levels at 60 minutes after the onset of stimulation; this increase was maintained for 3 hours after stimulation cessation (P < 0.05, vs. SNL group; Figure 2A). The SNL group had significantly increased expression of P62 and LC3-II (P < 0.05, vs. sham group; Figure 2B–D), which was partly reversed by HF-SCS treatment (P < 0.05, vs. SNL group; Figure 2B–D).
Figure 3|Effects of HF-SCS on the protein expression of LC3 and P62 in the ipsilateral L4–5 spinal dorsal horn of neuropathic pain rats with CQ application.

The results of Experiment 2 showed that, after CQ application, the levels of both LC3-II and P62 were markedly increased in the sham-SNL rats (P < 0.05, vs. sham + vehicle group; Figure 3A–C). This finding reflects the normal status of autophagosomal degradation in the spinal cord of rats under physiological conditions. However, there were no significant differences in the expression of LC3-II or P62 in the SNL rats undergoing sham-HF-SCS between those that received CQ and those that did not (P > 0.05; Figure 3D–F), suggesting that spinal autophagosome degradation was already defective in the SNL rats. In contrast, the application of CQ caused a marked accumulation of LC3-II and P62 in the SNL rats receiving HF-SCS treatment (P < 0.05, vs. SNL + HF-SCS + vehicle group; Figure 3G–I). This finding indicates that HF-SCS treatment can restore autophagosome degradation and alleviate the impairment of autophagic flux in SNL rats.
Figure 4|Effects of HF-SCS on lysosomal dysfunction in the spinal dorsal horn of neuropathic pain rats.

Figure 5|HF-SCS attenuates SNL-induced lysosomal dysfunction in neuropathic pain rats, mainly in the neurons of the superficial layers of the ipsilateral dorsal horn.
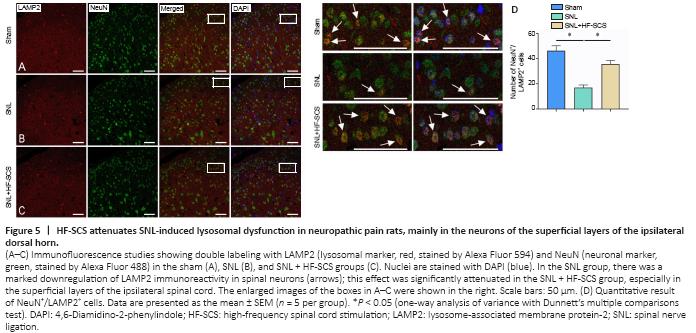
The western blot results of Experiment 3 revealed a significant decrease in LAMP2 and the mature form of CTSD (matu-CTSD) in the SNL group (P < 0.05, vs. sham group; Figure 4A–C), indicating that both lysosomal quantity and activity were decreased in the SNL group. However, HF-SCS treatment increased the levels of LAMP2 and matu-CTSD in the spinal horn dorsal cord of never-injured rats (P < 0.05, vs. SNL group; Figure 4A–C). The confocal immunofluorescence results demonstrated that spinal LAMP2 was primarily co-localized with the neural marker NeuN (Jongen-Rêlo and Feldon, 2002). Furthermore, HF-SCS treatment significantly attenuated the SNL-induced downregulation of LAMP2 immunoreactivity in spinal neurons, especially in neurons in the superficial layers of the ipsilateral dorsal horn (P < 0.05, vs. SNL group; Figure 5A–D).
Figure 6|HF-SCS induces a long-lasting analgesic effect that can be inhibited by CQ pretreatment via the blocking of autophagic flux in the spinal cord of neuropathic pain rats.
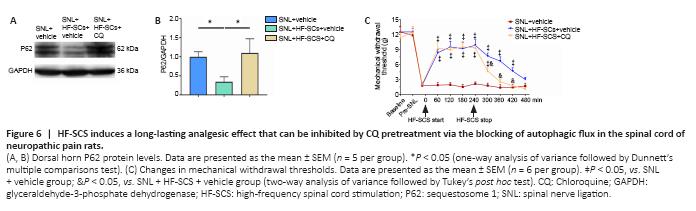
The results of Experiment 4 revealed that the HF-SCS-induced decrease of the abnormally elevated levels of P62 in SNL rats was significantly reversed when CQ was applied (P < 0.05, SNL + HF-SCS + CQ group vs. SNL + HF-SCS + vehicle group; Figure 6A and B), indicating that inhibition by CQ had an impact on autophagy in the spinal cord of rats of SNL + HF-SCS + CQ group, as expected. Furthermore, during HF-SCS treatment, the MWTs of rats who had received pretreatment with CQ were not significantly different from those without CQ treatment at 60–240 minutes after stimulation cessation (P > 0.05; Figure 6C). However, once the HF-SCS instrument was turned off, the CQ-induced inhibition of autophagy was able to rapidly reduce the restored MWTs of nerve-damaged rats, at 300–420 minutes after stimulation cessation (P < 0.05, SNL + HF-SCS + CQ group vs. SNL + HF-SCS + vehicle group; Figure 6C). In addition, the MWTs of SNL + HF-SCS + CQ group were not significantly different compared with the SNL group at 360–480 minutes after stimulation cessation (P > 0.05; Figure 6C).