周围神经损伤
-
Figure 1|Morphological analysis of axons in a peripheral nerve ECM-derived environment.

DNM-G was precoated at the bottom of the culture dishes, followed by DRG tissue seeding. Matrigel-precoated dishes were included as controls. After 72 hours of incubation, the axons growing on the DNM-G substrate were clustered into large bundles, whereas the Matrigel gave rise to dispersed and curved axons (Figure 1A). The axonal trajectories on the other ECM substrates (laminin 521, collagen I, and collagen IV) were also in a dispersed state (Additional Figure 1). Axon bundle diameter analyses (Additional Figure 2) showed that more than 20% of the axon bundles in the DNM-G were larger than 3 μm in diameter, an d that 40% were between 1 and 3 μm. In the Matrigel group, we detected no axon bundles larger than 3 μm; more than 80% of the axon bundles were less than 1 μm in diameter (n = 5 cultures, P < 0.0001, Figure 1B).
The interactions between axons in transverse sections were examined via transmission electron microscopy. We found that there were more axons in contact with each other in the DNM-G group (72.42 ± 8.03%) than in the Matrigel group (18.01 ± 5.52%, n = 6 experiments, more than 90 axons were counted in each experiment, P < 0.0001, Figure 1C and D).
Figure 2|NCAM1 mediates the clustering of neurites.
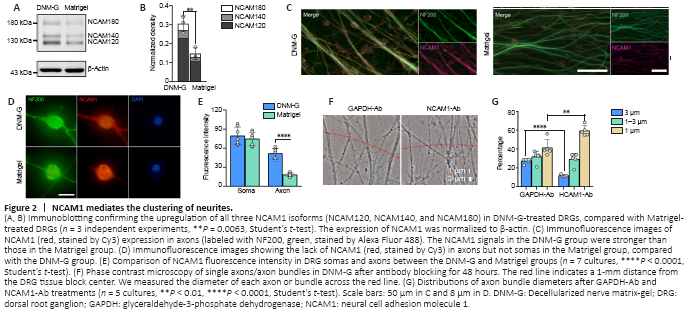
CAMs are known to regulate a series of cell dynamics. Among them, NCAM1 was selected as the putative candidate owing to its role in regulating axonal fasciculation (Cunningham et al., 1987; Lin et al., 1994; Cremer et al., 1997). Immunoblotting confirmed the upregulation of three NCAM1 isoforms (NCAM180, NCAM140, and NCAM120) in DNM-G-treated DRGs compared with Matrigel-treated DRGs (P = 0.0063, Figure 2A and B). We also tracked dynamic changes in NCAM1 expression levels and found an increase in NCAM1 after DNM-G treatment, in contrast to a decrease after Matrigel treatment (P < 0.0001, Additional Figure 3A and B).
Consistent with the immunoblot results, immunofluorescence staining of both DRG ex vivo (Figure 2C) and DRG neuron cultures showed higher axonal NCAM1 fluorescence intensity in the DNM-G group compared with the Matrigel group (P < 0.0001, Figure 2D and E). However, the fluorescence intensity of NCAM1 in the DRG soma was not significantly different between the two groups (P = 0.4698, Figure 2D and E). These results indicate that the DNM-G-induced axonal fasciculation was correlated with the expression of NCAM1 in axons.
We blocked the function of NCAM1 using an antibody that specifically binds to its extracellular region. We used an IgG that binds to cytoplasmic GAPDH and has no biological function outside the cell as a negative control (Figure 2F). In comparison with the GAPDH antibody, the NCAM1 antibody treatment reduced the diameter of the axon bundles (Figure 2G). These data confirmed the role of NCAM1 in mediating axonal fasciculation.
Figure 3|Identification of COL6 as the extracellular signal initiating axonal fasciculation.
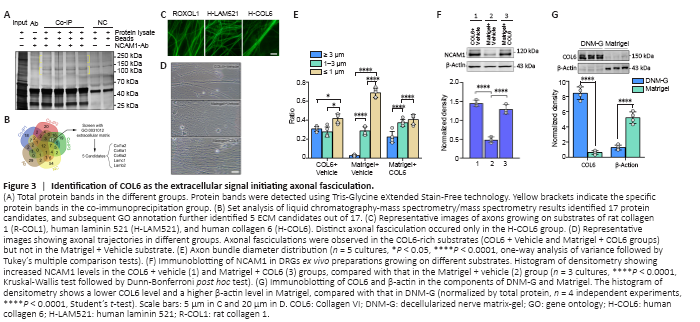
Interactions between NCAM1 and ECM components are highly relevant to cytoskeletal rearrangements, and thereby modulate a range of biological processes, including cell adhesion and migration (Nielsen et al., 2010). To assess the presence of interactions between NCAM1 and DNM-G components, we performed co-immunoprecipitation in the DNM-G-DRG culturing system using NCAM1 as bait. Sodium dodecyl sulfate polyacrylamide gel electrophoresis showed several specific bands in the co-immunoprecipitation lanes (Figure 3A). High-resolution liquid chromatography-mass spectrometry/mass spectrometry identified the protein species in these bands. The proteins in the NCAM1-Ab solution and in the negative control (NC) were also found to exclude nonspecific binding proteins. We screened 17 proteins by analyzing the liquid chromatography-mass spectrometry/mass spectrometry data, and further reduced the number of protein candidates to five (COL1A2, COL6A1, COL6A2, LAMC1, and LAMB2) by selecting those belonging to the ECM (Figure 3B). These five protein monomer candidates are included in three protein complexes: collagen I (COL1), COL6, and laminin 521 (LAM521).
We then tested these protein complexes in DRG ex vivo preparations. We found that commercially available human COL6 (H-COL6) could induce orderly axon bundle formation, whereas commercially available rat collagen I (R-COL1) and human-Laminin 521 (H-LAM521) could not (Figure 3C). The commercial H-COL6 protein was in the pepsin-resistant triple helical fragment form, and was not contaminated with fibronectin (data not shown).
Moreover, the addition of COL6 (20 μg/mL) to Matrigel (1 mg/mL) promoted axon bundle formation (Figure 3D and E) and upregulated the expression of NCAM1 at a level comparable to that of the COL6 + Vehicle group, which was approximately three times that in the Matrigel + Vehicle group (Figure 3F). The immunofluorescence results also showed an increased axonal NCAM1 fluorescence intensity in the COL6-vehicle and Matrigel-COL6 groups compared with that in the Matrigel-Vehicle group (Additional Figure 4). These results pointed at COL6 as the extracellular signal initiating axonal fasciculation, and were consistent with the immunoblot data showing the lack of COL6 in Matrigel components compared to DNM-G components (Figure 3G).
Figure 4|COL6-NCAM1 interaction inhibits lysosomal degradation of NCAM1.
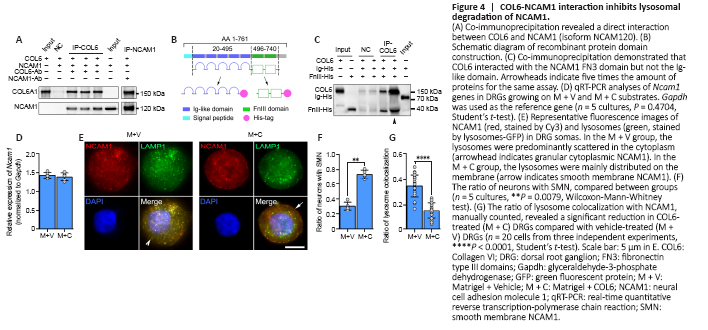
The co-immunoprecipitation-derived protein mass spectrometry data above revealed the interaction between COL6 and NCAM1. Although the co-localization of COL6 and NCAM1 immunofluorescence in the COL6-treated DRG ex vivo sample indicated a connection between NCAM1 and COL6 (Additional Figure 6), this connection could be direct or indirect, as many other proteins bind them. To exclude any indirect interactions caused by other proteins, we performed immunoprecipitation on a solution containing purified NCAM1 (human isoform NCAM120) and COL6. Using COL6 or NCAM1 as the bait protein, we found that NCAM1 or COL6 could be coimmunoprecipitated and detected by subsequent immunoblotting (Figure 4A). The extracellular part of NCAM1 comprises an Ig-like domain and a fibronectin type III-homology domain, and each determines different functions of NCAM1 (Ranheim et al., 1996; Carafoli et al., 2008). Thus, we prepared two recombinant protein segments, one containing five Ig-like domains and the other containing the FN3 domains (Figure 4B). According to the co-immunoprecipitation results, when we incubated COL6 with these two protein segments as bait, only the FN3-containing segment could be detected by subsequent immunoblotting (Figure 4C).
We next questioned how the binding of NCAM1 with extracellular COL6 can upregulate the expression of NCAM1 in axons, since our real-time polymerase chain reaction showed no increase in the transcription of the Ncam1 gene in COL6-treated DRGs (Figure 4D). Clues from immunofluorescence staining showed two distinct distribution patterns of NCAM1 in neurons: a granular distribution in the cytoplasm (Figure 4E) and a smooth distribution on the membrane (Figure 4E). The ratio of neurons with smoothly distributed NCAM1 on the membrane was 0.74 ± 0.06 in the COL6 treatment group (M + C), which was higher than that in the vehicle treatment group (M + V, 0.31 ± 0.05, n = 5 cultures, P = 0.0079, Figure 4F). NCAM1 on the cell membrane is reportedly downregulated through endocytosis and the lysosomal degradation pathway (Diestel et al., 2007; Wobst et al., 2012). Therefore, we examined NCAM1 degradation activity via double fluorescence labeling of NCAM1 and lysosomes. Lysosomes-Green Fluorescent Protein vector labeling showed that the ratio of lysosome colocalization with NCAM1 to the total number of lysosomes was significantly reduced in COL6-treated neurons (0.16 ± 0.06) compared to vehicle-treated neurons (0.35 ± 0.09, n = 20 cells, P < 0.0001, Figure 4G).
Figure 5|Addition of COL6 in Matrigel improves structural and functional recovery of sciatic nerve defect.
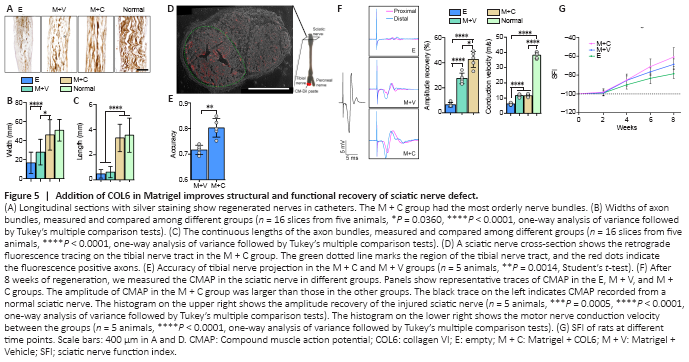
After 8 weeks of regeneration, the nerve stumps were well connected with regenerated tissue inside the catheter, and the regenerated tissue in the M + C group had the largest diameter (Additional Figure 7B and C). Silver staining revealed a certain amount of disorganization among axons in the E and M + V groups. In contrast, in the M + C group, the axons were clustered into bundles that resembled the structure in normal sciatic nerves (Figure 5A). Morphological analysis revealed that the width and continuous length of the axon bundles were significantly increased in the M + C group compared with the E and M + V groups (P < 0.0001, Figure 5B and C).
Moreover, we analyzed the accuracy of nerve bundle projections via retrograde fluorescence tracing of the tibial nerve tract (Figure 5D and Additional Figure 8B). After 5 days of tracing, the accuracy of the tibial nerve projection in the M + V group was lower than that in the M + C group (Figure 5E, 0.72 ± 0.02 vs. 0.81 ± 0.04, n = 5, P = 0.0014).
Functionally, after 8 weeks of repair, the empty silica catheter bridging (E) only resulted in a 6.42 ± 1.78% increase in sciatic nerve CMAP. Filling the silicone catheter with 10 mg/mL Matrigel (M + V) enhanced the recovery of CMAP to 27.46 ± 4.22%, and the addition of 20 μg/mL COL6 to Matrigel (M + C) further increased the recovery of CMAP to 43.05 ± 6.75%. The motor nerve conduction velocities were not significantly different between the M + V and M + C groups (Figure 5F). The sciatic nerve function index data also showed the highest functional recovery level in the M + C treatment group (–60.91 ± 9.89), compared with that in the M + V (–68.82 ± 5.78) and E (–78.66 ± 5.15, n = 5, Figure 5G) groups.
Figure 6|Mechanisms of COL6-initiated nerve bundle formation.
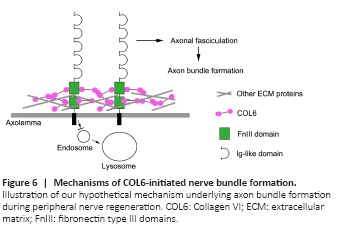
Taken together, the results of this study revealed that the binding of extracellular COL6 to the NCAM1 FN3 domain prevents the lysosomal degradation of NCAM1, resulting in the accumulation of NCAM1 on the axolemma. Subsequently, the NCAM1 molecules on the axolemma promote axonal fasciculation that facilitates the formation of nerve bundle structure (Figure 6).