脊髓损伤
-
Figure 1|Reduction of ALDH2 levels post SCI and its influence by Alda-1.
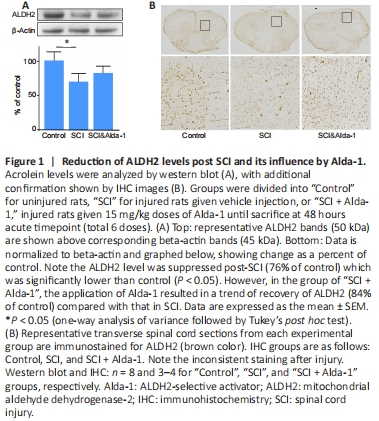
We first tested the hypothesis that the protein expression level of ALDH2 is reduced in SCI, a situation known to have elevated levels of acrolein. As shown in Figure 1, western blot analysis showed a significant decrease in ALDH2 expression when examined 2 days following a contusive spinal cord injury (76% of control). Such reduction is significant when compared with control (P < 0.05, ANOVA). Treatment of Alda-1, an ALDH2 stimulator, resulted in a trend of recovery of ALDH2 (84% of control) compared with that in SCI (Figure 1A). In Figure 1B, in the control group, strong ALDH2 staining was and its organized pattern in both grey and white matter were observed. In both SCI and SCI + Alda-1 groups, ALDH2 appeared less organized with lighter staining.
Figure 2|Co-immunoprecipitation indicating acrolein-bound-ALDH2 in vivo after SCI.
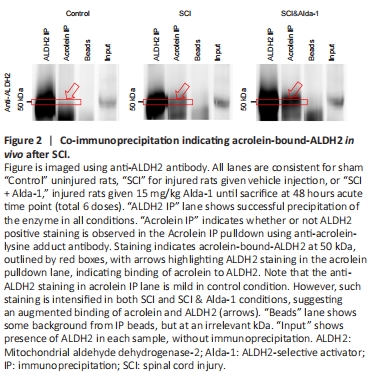
Aldehydes are highly reactive compounds and capable of adducting to proteins, such as ALDH2. In fact, at high concentrations, aldehydes are known to inhibit ALDH2, likely by this mechanism (Ferencz-Biro and Pietruszko, 1984; Mitchell and Petersen, 1988; Ren et al., 1999; Doorn et al., 2006; Yoval-Sanchez and Rodriguez-Zavala, 2012). As such, we then examined the possibility that the binding of acrolein to ALDH2, a likely step of acrolein-induced ALDH2 inhibition, was elevated in SCI, using a co-immunoprecipitation (co-IP) technique. As indicated in Figure 2, ALDH2 precipitated at 50 kDa as expected (ALDH2 IP lane). Arrows indicate that acrolein-bound ALDH2 is present in both injury and Alda-1 treated groups. Some background from beads was observed but did not interfere with the 50 kDa ALDH2 bands. Confirmation of successful co-immunoprecipitation of acrolein is further shown in Additional Figure 1.
Figure 3|Analysis of acrolein levels post-SCI.
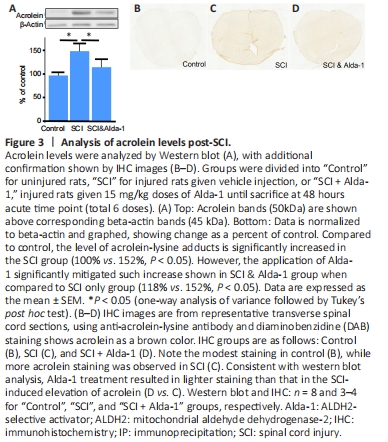
However, such elevated acrolein levels can be significantly suppressed to 118% of control when injured animals were treated with Alda-1, which was significant when compared to SCI only (P < 0.05). Consistent with western blot analysis, representative sections of spinal cord with DAB staining from Control, SCI, and SCI + Alda-1 groups reflects these findings. Specifically, acrolein level (brown color) was elevated following SCI, while Alda-1 could suppress post-SCI acrolein elevation. As such, the findings from both western blot and immunohistochemical staining suggest that ALDH2 enhancement could lower the post-SCI acrolein surge, indicative of a critical role of ALDH2 in post-SCI acrolein elevation (Figure 3A–C).
Figure 4|Alda-1 treatment reduces cyst formation in the spinal cord post-injury.

Acrolein is an established secondary injury mechanism known to cause severe structure damages. Furthermore, lowering the acrolein using acrolein scavengers could reduce spinal cord cyst, a common pathology observed after contusion injury, resulting from widespread cell death, and a key indication of post-SCI tissue destruction (Park et al., 2014; Gianaris et al., 2016). Therefore, we predicted that more effective ALDH2 could reduce acrolein and lead to reduced cyst area. As indicated in Figure 4, no clear cyst is visible in control cord (Figure 4A). However, SCI has resulted in conspicuous cyst formation in the injury site (Figure 4B). Again, the application of Alda-1 markedly reduced the size of the cyst (Figure 4C). Quantitative analysis revealed a significant decrease in cyst size with Alda-1 treatment compared with SCI (Figure 4D) (P < 0.05).