周围神经损伤
-
Figure 1|Single cell sequencing of rat dorsal root ganglion neurons.

The DRG cells, harvested after establishing the crush model, were subjected to RNA sequencing by Smart-seq2. The sequence data underwent quality control to obtain clean data. The workflow is shown in Figure 1A. Although the number of single DRG neurons obtained by LCM was 100 to 200 at each time point, only 100 repeats at each time point are needed to undergo scRNA-seq2 sequencing.
We obtained the differentially expressed genes (DEGs) by comparing the expression of genes in the injury groups (3, 6, 12 hours, 1, 3 and 7 days) with the normal group (0 hour). A total of 1228 genes showed different expression after injury (|log2FC| ≥ 1 and P-value ≤ 0.05), as shown in Figure 1B, with red representing up-regulated genes, green representing down-regulated genes and gray representing genes with no significant differences (Figure 1B). According to a previous report (Wang et al., 2020), axonal regenerative repair started from 3 days after PNI. From 1 day after the injury, genetic reprogramming was induced intensely and lasted until the 7th day (Renthal et al., 2020). Therefore, the genes with altered expression in each of these three groups may have an important influence on axon regeneration. We found 189 common DEGs by intersection of 391 DEGs at 1 day (0 hour vs. 1 day), 795 DEGs at 3 days (0 hour vs. 3 days) and 771 DEGs at 7 days (0 hour vs. 7 days) (Figure 1C).The interaction between these 189 DEGs was explored with String database. After filtering out the molecules that had no relationship with others, the remained relationships are shown in Figure 1D. The molecules in the center of the network present more relationships with others, indicating they may have more influence on this network. The core molecules include a number of cytokines or growth factors, such as Il6, Ngf, Fgf2, Gfap, Cd44, and Ccl2. and transcription factors or regulators such as Atf3, Jun, Myc, Crem, Socs3, and Ezh2.
Figure 2|Expression of Ezh2 in dorsal root ganglion neurons.

To investigate the role of Ezh2 in the repair of PNI, we first verified the expression of Ezh2 in DRG neurons. Our scRNA-seq results show that Ezh2 expression consistently increased in L4–5 DRG neurons (Figure 2A). Our RT-qPCR, FISH and immunofluorescence experiments showed that Ezh2 expression was consistently increased in injured DRG neurons (Figure 2B–E).
Figure 3|Expression of Ezh2 in NF160/200- and IB4-positive dorsal root ganglion neurons after sciatic nerve injury.

In DRG neurons immunofluorescence experiments, we found that the expression of Ezh2 differed in different sizes of DRG neurons. Hu et al. (2016) reported that the heterogeneity of DRG neurons resulted in different sizes of neurons with different sensory functions. Therefore, we labeled DRG neurons by IB4 (small) and NF160/200 (large) (Usoskin et al., 2015). We found that the expression of Ezh2 also increased in large- and small-sized neurons after injury. Moreover, the expression of Ezh2 was different between large- and small-sized neurons, and there were many NF160/200-positive neurons (Figure 3). In conclusion, the expression of Ezh2 during the repair of PNI was continuously increased, and the expression of Ezh2 in NF160/200-positive large-sized neurons was high.
Figure 4|Functional verification of Ezh2 in cultured dorsal root ganglion neurons.

We have shown above that the expression of Ezh2 in DRG was consistently increased after PNI. To explore the function of Ezh2 in DRG neurons, we cultured DRG neurons in vitro. First, we transfected neurons with siRNA of Ezh2 and screened the knockdown efficiency of siRNA on Ezh2 expression (Figure 4A), then we stained the neurite of DRG neurons with TuJ1. The staining results indicate that the lengths of neuron neurites in the three groups transfected were significantly shorter than the control group (Figure 4B), including the mean length and the longest length (Figure 4C and D). These results suggest that the knockdown of Ezh2 expression can inhibit the axonal growth of DRG neurons. Counts of the distribution of neurite lengths showed that the percentage of neurite lengths < 400 μm was significantly higher in Ezh2-silenced DRGs than that in the normal control group, whereas the percentage of neurite length > 400 μm was lower in Ezh2-silenced DRGs than that in the normal control group (Figure 4E). This indicated that the neurite growth was significantly inhibited after silencing Ezh2. Finally, we examined the axon growth statistics of DRG neurons after Ezh2 knockdown in large-sized neurons (surface area > 800 μm2) and small-sized neurons (surface area < 800 μm2), which revealed that the neurite growth was significantly suppressed in both (Figure 4F). All these results indicate that Ezh2 is important for DRG neuron regeneration.
Figure 5| Ingenuity pathway analysis predicts the upstream and downstream interaction factors of Ezh2.
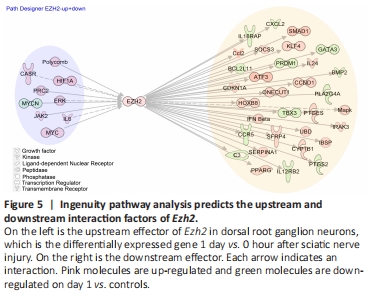
Ezh2 is very important for DRG neuron regeneration. Inhibition of Ezh2 expression inhibits the growth of DRG axons. However, it is not clear how Ezh2 works or what the possible mechanism and reaction network are. To further search for the regulatory mechanism of Ezh2 involved in axonal growth, we used Ingenuity Pathway Analysis (IPA) to intersect these molecules with our 1-day vs. control differential genes. We found 28 up-regulated regulators and 13 down-regulated effectors of Ezh2 (Figure 5). The different shapes in the graph represent different molecule types. Among the many molecule types, upstream factors such as CASR and MYC have been found to play a role in neuronal axon growth. We hope that the possible upstream and downstream factors of Ezh2 predicted by us in Figure 5 will provide the basis for the follow-up studies on PNI repair.
点击此处查看全文