脊髓损伤
-
Figure 3| Effect of SS on hemolysis and ferroptosis-specific mitochondrial changes after spinal cord injury.
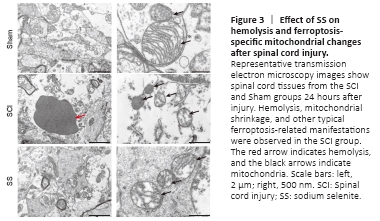
We evaluated the tissue ultrastructure in transverse sections of spinal cords 24 hours post-injury by transmission electron microscopy (Figure 3). In the SCI group, hemolysis was visible in the spinal cord tissue, and typical manifestations of ferroptosis were observed, including shrunken mitochondria, increased membrane density, and occasionally disrupted outer membranes. Hemolysis and characteristic manifestations of ferroptosis in the SS group were markedly decreased when compared with that in the SCI group, whereas none of these features were identified in the Sham group. Neither the SS group nor the SCI group exhibited typical manifestations of apoptosis, such as apoptotic bodies.
Figure 5|Possible mechanisms by which SS improve GPX4 expression after SCI.
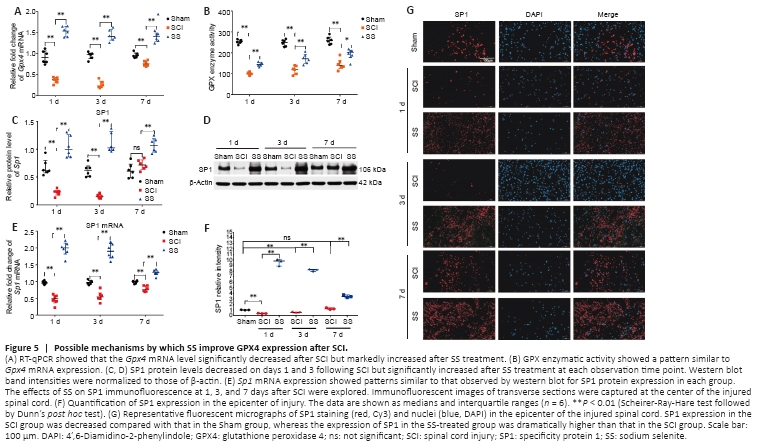
We determined the biomechanism by which SS promoted GPX4 expression and inhibited ferroptosis. Sp1 and Gpx4 mRNA and protein expression and GPX enzymatic activity was analyzed on days 1, 3, and 7 after SCI. Relative to expression in the Sham group, the SCI group showed markedly lower GPX4 protein and Gpx4 mRNA expression (Figures 4H, I and 5A). GPX activity was also reduced by injury (Figure 5B). SS treatment led to marked increases in these indicators.
Western blot, RT-qPCR, and immunofluorescent staining revealed that SP1 protein and mRNA expression was decreased after SCI, most noticeably within 3 days, and SS treatment improved the expression of these molecules substantially at all three time points post-injury (Figure 5C–G). Taken together, SS may upregulate GPX4 expression by increasing the expression of the transcription factor SP1, thereby enhancing GPX4 activity, suppressing ferroptosis, and promoting neurological recovery after SCI.
Figure 6|Effects of SS treatment on spinal tissue repair after SCI.
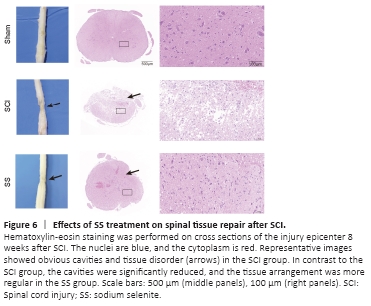
Hematoxylin-eosin staining of spinal cord tissues at 8 weeks post-injury is shown in Figure 6. The structures of spinal cord tissues in the Sham group were basically normal, whereas the SCI group displayed spinal cord tissue breakdown, scar connections, structural disorder, and obvious cavity formation. Comparatively, the cavities within the spinal cord tissues of the SS group were significantly reduced, and tissue arrangement demonstrated greater regularity. Thus, we inferred that SS promoted repair of injured spinal cord tissue and, subsequently, improved hind limb motor function.
Figure 7|Effects of SS on the numbers of NeuN+, GFAP+, and CNP+ cells after SCI.
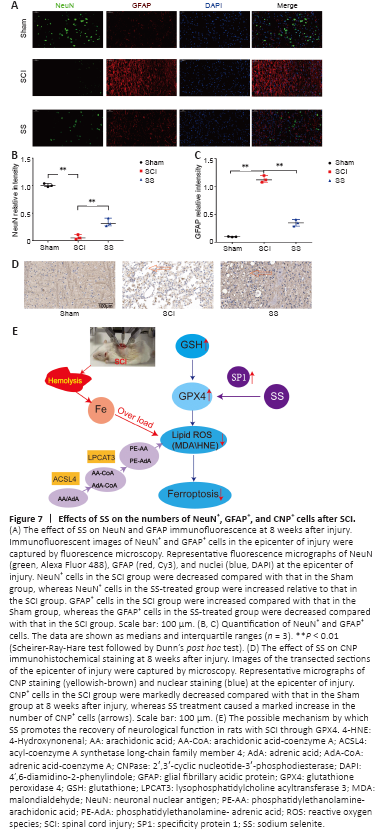
We performed immunofluorescent staining and immunohistochemical analysis to identify potential target cells that may benefit from SS treatment in rats with SCI. Immunofluorescent staining revealed that the numbers of NeuN+ cells in the SCI group were significantly decreased relative to that in the Sham group at 8 weeks post-injury, whereas the numbers of NeuN+ cells in the SS group were increased compared with that in the SCI group. In comparison, the numbers of GFAP+ cells in the SCI group were substantially higher relative to those in the Sham group at 8 weeks after injury, whereas the SS group showed a slight increase (Figure 7A–C). The differences between the SCI and SS groups were statistically significant for both proteins.
Immunohistochemical results demonstrated that the numbers of CNP+ cells in the SCI group were markedly decreased compared with those in the Sham group at 8 weeks after injury (Figure 7D). SS treatment caused a substantial increase in the numbers of CNP+ cells compared with that in the SCI group. Because CNP is a specific marker of oligodendrocytes and myelinated fibers (Müller and Seifert, 1982), we concluded that SS treatment protected oligodendrocytes during the pathology of SCI.
In the ICH model, Alim et al. (2019) found that human brain cells autonomously regulated cell damage caused by hemorrhage through Se intake. Further studies showed that selenium upregulated the expression of GPX4, inhibited ferroptosis, and protected neurons through activation of the transcription cofactor SP1. The 3? untranslated region of GPX4 mRNA contains a selenocysteine insertion sequence element (Touat-Hamici et al., 2014). During translation, the UGA codon is usually read as the termination codon, while in the presence of the selenocysteine insertion sequence, the UGA codon encodes an active site selenocysteine (U46). This particular form of translation requires a unique protein system to guide the insertion of selenocysteine into GPX4 and other selenoproteins. Therefore, the expression of GPX4 is regulated by the availability of selenium (Min et al., 2018). Both SCI and ICH are central nervous system injuries; therefore, we speculated that selenium functioned via a similar mechanism in SCI. Our results suggest that SS may increase the levels of the transcription factor SP1 and upregulate the expression of GPX4, thus inhibiting ferroptosis (Figure 7E).