视神经损伤
-
Figure 1|Mitochondrial distribution visualized on whole mounts and cryosections of MitoeGFP+ retinas, harvested at baseline (naive) or after ONC injury.

To first decipher whether mitochondria translocate within the different compartments of RGCs during their spontaneous injury-induced regrowth in adult zebrafish, mitochondrial distribution was studied using retinal whole mounts of naive and injured MitoeGFP zebrafish throughout the different regeneration phases: (1) the dendritic retraction phase (1–3 dpi), (2) the axonal regrowth phase (3–6 dpi) and the dendritic regrowth phase, occurring upon target reinnervation (6–14 dpi). Confocal z-stack images revealed GFP+ mitochondria in the IPL, RGCL, and NFL of naive retinas. Upon ONC, mitochondrial quantities decreased in the IPL from 3–10 dpi, whereafter they restored to baseline levels (Figure 1A). In the RGCL, a strong increase in mitochondrial number and a more scattered mitochondrial distribution were observed at 3 dpi. This spread-out mitochondrial arrangement remained observable in the RGCL until ±14 dpi. Three weeks after injury, the mitochondrial labeling in the RGCL was comparable to that of naive retinas (Figure 1B). No obvious changes in mitochondrial appearance were noted in the NFL throughout the regenerative process (data not shown).
Similar observations were made using sagittal cryosections of naive and injured eyes (Figure 1C). At 3 dpi, the number of mitochondria in the IPL was reduced, and minimal mitochondrial levels were reached at 6 dpi. Strikingly, at 3–6 dpi, mitochondria in the most inner IPL appeared as MitoEGP+ stripes sprouting from the RGC somas, presumably visualizing mitochondrial accumulation in the primary dendrites. A high magnification image in Additional Figure 4 shows that numerous mitochondria are present in the primary dendrite, visualized by the red membrane tag at 3 dpi, in contrast to what is observed in the control condition. Together with the reduced mitochondrial density in the outer IPL, these data suggest that mitochondria redistribute from random in the dendritic tree towards the RGC primary dendrite after injury. The number of mitochondria in the IPL remained low at 10 dpi, although a notable increase was detected in the most outer IPL, adjacent to the inner nuclear layer (INL). Afterwards, mitochondrial numbers raised again to reapproach baseline levels 2 to 3 weeks after injury (Figure 1C). In the RGCL, a transient rise in mitochondrial levels was observed at 3 dpi. In the NFL, no obvious changes in mitochondrial density were detected using the retinal cryosections.
Figure 3|Mitochondrial distribution visualized and quantified on cryosections of MitoeGFP+ visual systems, harvested at baseline (naive) or after ONC injury.
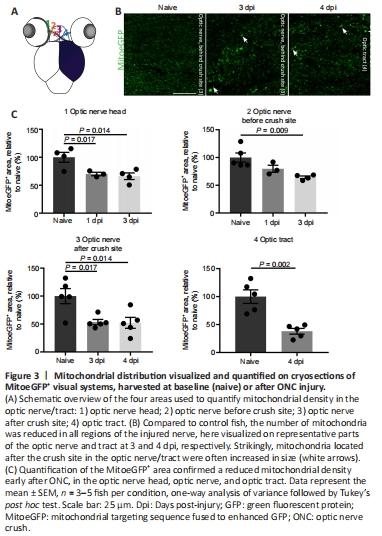
Hereafter, we specifically investigated mitochondrial appearance/density in the RGC axons at early time points after ONC using horizontal visual system sections of MitoeGFP zebrafish. Morphometric analyses were performed at four different locations: at the optic nerve head (1), in the optic nerve before (2) or after the crush site (3), and in the optic tract (4) (Figure 3A). As it is well known that the regenerating axons are located just past the site of impact at three days post-ONC in adult zebrafish (Beckers et al., 2019), we quantified mitochondrial area in the first two positions at 1 and 3 dpi. The mitochondrial area in the more distal axonal regions was assessed at 3 and 4 dpi, time points at which the (pioneering) axons are located in the optic tract but have not reached the optic tectum yet (Beckers et al., 2019). In general, and to our surprise, the MitoeGFP+ area was decreased at all locations after ONC injury, indicating that less mitochondria were present in the damaged optic nerve/tract as compared to the naive situation (Figure 3B and C). Notably, enlarged mitochondrial deposits were observed after the crush site in the optic nerve and optic tract at 1, 3, and 4 dpi (Figure 3B).
Figure 4|Mitochondrial distribution visualized on vibratome sections of MitoeGFP+ optic tecti, harvested at baseline (naive) or after ONC injury.
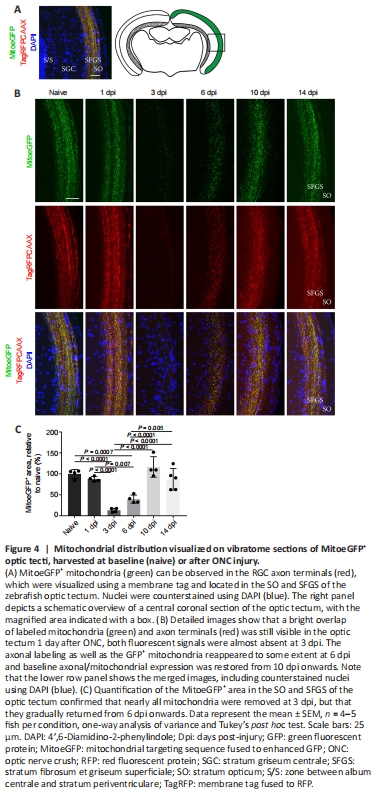
We also aimed to characterize mitochondrial distribution in the optic tectum at various time points after ONC using coronal optic tectum sections of MitoeGFP zebrafish (Figure 4A). To link mitochondrial presence with axonal degeneration and regeneration, we made use of the RFP+ membrane tag. At baseline, MitoeGFP+ mitochondria were clearly located inside the RFP+ axons, which remained unchanged until 1 day after optic nerve injury (Figure 4B and C). At 3 dpi, the mitochondrial signal, as well as the RFP fluorescence, was drastically decreased, possibly indicative of axonal degeneration. Both MitoeGFP+ and RFP+ labeling were found to slightly reappear at 6 dpi, and were fully restored to baseline levels from 10 dpi onwards, known to be the time point at which tectal reinnervation is completed in adult nerve-crushed zebrafish (Beckers et al., 2019). Thus, our findings suggest that, while RGC axonal degeneration in the tectal innervation areas coincides with the loss of mitochondria, axonal reinnervation is strongly linked with a return of these energy-producing organelles.
Figure 5|Immunofluorescent stainings for Pgc-1α, p-Drp1, Opa1, and Pink1 on cryosections of WT retinas, harvested at baseline (naive) or after ONC injury.
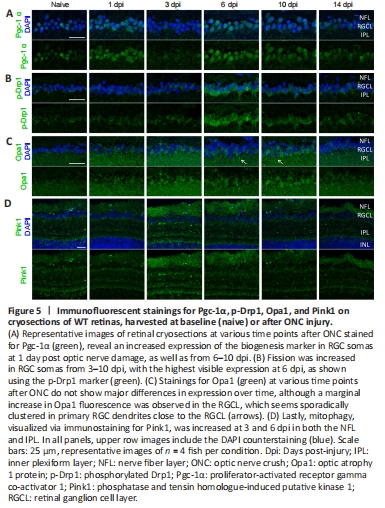
To substantiate our findings showing a differential mitochondrial distribution inside the retina after ONC, we next determined mitochondrial dynamic processes in the RGCs, including biogenesis, fission/fusion, and mitophagy, using immunofluorescent stainings for respective markers on retinal cryosections of WT zebrafish at different time points after ONC. Mitochondrial biogenesis was evaluated using Pgc-1α, a key regulator and well-known marker of this process. In naive fish, only a few RGCs were found positive for Pgc-1α, but increased mitochondrial biogenesis in RGC somas could be clearly detected at two defined time points post-injury, namely at 1 dpi and from 6–10 dpi (Figure 5).