视神经损伤
-
Figure 2|OGD/R-induced R28 cells exhibit pyroptosis, apoptosis, and necroptosis.
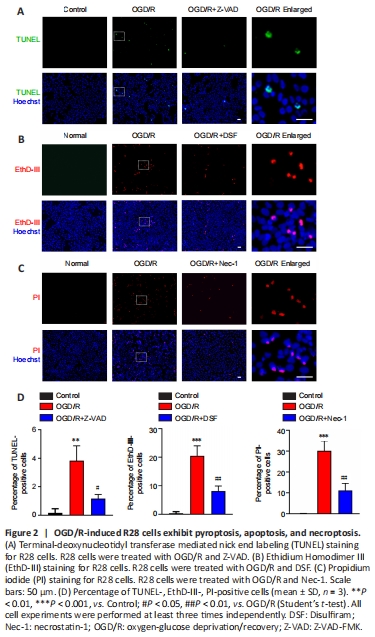
The OGD/R treatment and staining timeline were the same in each group. Cell staining results indicated that the OGD/R treatment significantly induced apoptosis (TUNEL staining; Figure 2A and D), pyroptosis (EthD-III staining; Figure 2B and D), and necroptosis (PI staining; Figure 2C and D). The RCD morphology of cells treated with OGD/R after pretreatment with inhibitors of apoptosis (Z-VAD; Figure 2A and D), pyroptosis (DSF; Figure 2B and D), and necroptosis (Nec-1; Figure 2C and D) was significantly reversed. These results indicated that PANoptosis-like cell death occurred in R28 cells following OGD/R injury, which is similar to those observed in PANoptosis in other models (Kuriakose and Kanneganti, 2019; Malireddi et al., 2019; Karki et al., 2021).
Figure 4|Combination of RCD inhibitors decreases the OGD/R-induced TUNEL-positive cells.

Figure 5|Combination of RCD inhibitors decreases the OGD/R-induced EthD-III-positive cells.
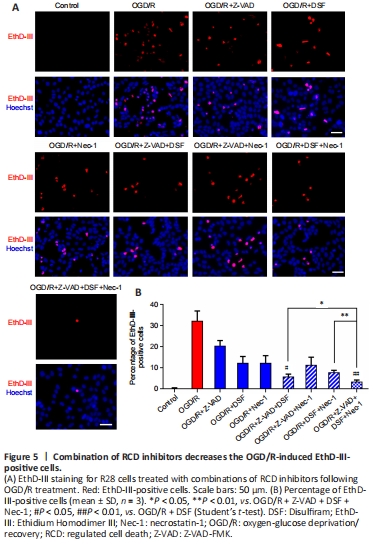
Figure 6|Combination of RCD inhibitors decreases the OGD/R-induced PI-positive cells.

The results above indicated that OGD/R treatment induced PANoptosis-like cell death in R28 cells at the same time point. Next, we investigated whether the cell loss following OGD/R treatment is mainly dependent on this kind of combined cell death. Thus, pretreatment with different combinations of inhibitors for apoptosis, pyroptosis, and necroptosis was used to assess the protective effects on cell loss against OGD/R treatment. As shown in Figure 4A and B, Z-VAD combined with either DSF or Nec-1 had a better protective effect than that of Z-VAD alone, as measured by TUNEL-positive cell death, following OGD/R treatment. There was no significant difference between the triple combination and the double combinations, and the TUNEL-positive cell death was largely reversed by the combined pretreatments. The combination of DSF with Z-VAD, or with both Z-VAD and Nec-1, had a better protective effect on EthD-III-positive cell death than DSF alone (Figure 5A and B). The triple combination of DSF, Z-VAD and Nec-1 had the largest protective effect on EthD-III-positive cell death compared with each of the double combinations. PI staining also indicated that the combination of Nec-1 with Z-VAD, or with both Z-VAD and DSF, had a larger protective effect than Nec-1 alone (Figure 6A and B). Moreover, the triple combination of Nec-1, Z-VAD and DSF had a larger protective effect on PI-positive cell death than the double combination of Nec-1 and DSF. Taken together, these results suggested that OGD/R-induced R28 cell death is mainly driven by PANoptosis-like cell death.
Figure 7|aHIOP induces pyroptosis, apoptosis, and necroptosis in vivo in rat retina.
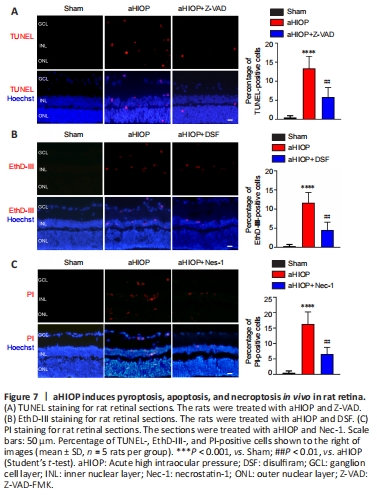
Figure 8| aHIOP induces high expression of pyroptosis, apoptosis, and necroptosis-related proteins in rat retina.
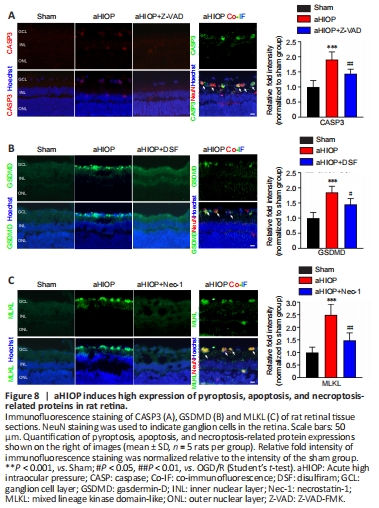
The above experiments indicated that OGD/R injury can induce PANoptosis-like cell death in vitro. Next, we used a rat aHIOP model to investigate whether I/R injury can induce PANoptosis-like cell death in vivo (Figure 1). As shown in Figure 7A, aHIOP treatment induced apoptotic cell death of retinal neurons (indicated by TUNEL staining) in the ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL). The increased TUNEL staining was significantly reduced by Z-VAD pretreatment. Increased caspase-3 expression was detected by immunofluorescence staining in retinal neurons, which further indicated the occurrence of apoptosis in retinal neurons following aHIOP treatment (Figure 8A). Furthermore, the co-immunofluorescence staining of caspase-3 and NeuN indicated that caspase-3 was activated in the retinal ganglion cells (RGCs) of aHIOP-treated retina.
The existence of pyroptosis in retinal neurons was indicated by EthD-III-positive staining in GCL and INL of aHIOP-treated retina (Figure 7B). EthD-III-positive staining in the retina was significantly reduced by pretreatment of specific pyroptosis inhibitor DSF. Moreover, as shown in Figure 8B, aHIOP treatment significantly increased GSDMD expression (indicated by immunofluorescence staining) in the GCL and INL, which was consistent with the EthD-III staining. Also, the co-immunofluorescence staining of GSDMD and NeuN indicated the activation of GSDMD in RGCs. Thus, these results indicated that aHIOP treatment induced pyroptosis in retinal neurons in vivo.
Similarly, aHIOP treatment increased PI staining, suggesting that aHIOP treatment induced necroptotic cell death, in the GCL, INL, and ONL of the retina, and Nec-1 pretreatment significantly reduced the PI-positive staining (Figure 7C). Furthermore, after aHIOP treatment, immunofluorescence staining showed increased expression of MLKL, a key protein of necroptosis execution, indicating that aHIOP treatment induced retinal cell necroptosis (Figure 8C). The co-immunofluorescence staining of MLKL and NeuN indicated the activation of MLKL in RGCs of aHIOP-treated retina. Taken together, the results demonstrated that PANoptosis-like cell death occurred in vivo in retinal neurons following aHIOP injury.
点击此处查看全文