周围神经损伤
-
Figure 2|Increased expression of miR-221-3p and miR-338-3p in proximal and distal nerve stumps at different time points after nerve transection.
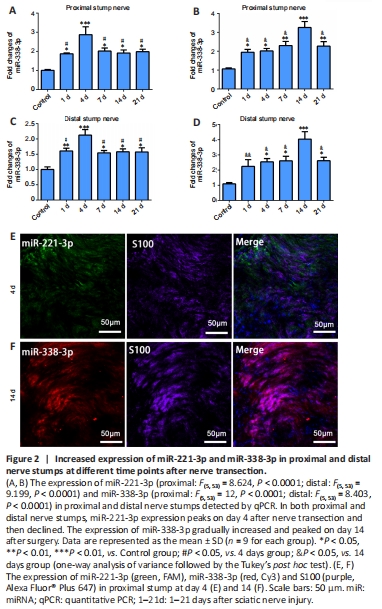
Rats with sciatic nerve defect as nerve injury models were used to investigate the expression and localization of miR-221-3p and miR-338-3p in injured sciatic nerves. qPCR was used to detect the expressions of miR-221-3p and miR-338-3p in the proximal and distal stumps of sciatic nerves at 0, 1, 4, 7, 14, and 21 days after nerve transection. miR-221-3p and miR-338-3p were expressed in both proximal and distal stumps and peaked at 4 and 14 days, respectively, after nerve transection (Figure 2). FISH in combination with immunostaining was used to detect the location of miR-221-3p and miR-338-3p in the proximal stumps of sciatic nerves at 4 and 14 days after nerve transection. The results showed that most miR-221-3p and miR-338-3p were expressed in cells positive for S100 (a marker of SCs) (Figure 2), which suggested that the expression of miR-221-3p and miR-338-3p of SCs increased after nerve injury.
Figure 3|miR-221-3p is involved in axonal elongation and the proliferation and migration of SCs in vitro, and miR-338-3p participates in the myelination of SCs in vitro.
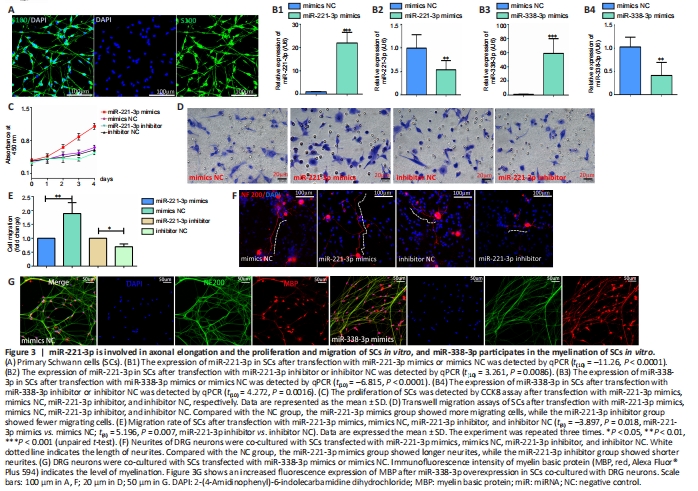
To investigate the functions of miR-221-3p in vitro, miR-221-3p mimics and inhibitor were transfected into primary SCs. Primary SCs exhibit spindle morphology and express S100, with a purity of up to 98% (Figure 3A). Results in Figure 3B1 and B2 confirmed that miR-221-3p mimics (P < 0.0001) and inhibitor (P = 0.0086) increased and inhibited the expression of miR-221-3p in SCs, respectively. CCK8 assays showed that the proliferation rate of SCs after overexpression of miR-221-3p was increased compared with that of other groups (Figure 3C). Transwell assays show that miR-221-3p mimics significantly promoted the migration of SCs, while miR-221-3p inhibitor decreased the migration capacity (Figure 3D and E). Moreover, co-culture of primary SCs and DRG neurons showed that miR-221-3p mimics increase the length of the neurite, while miR-221-3p inhibitor decreased the length of neurite in comparison with the mimics NC group (Figure 3F). These findings indicate that miR-221-3p promotes proliferation and migration of SCs and accelerates the neurite outgrowth.
qPCR results confirmed that miR-338-3p expression was upregulated or downregulated after miR-338-3p mimics or inhibitor transfection into SCs, respectively (Figures 3B3 and B4). The results in Figure 3G show an increased myelination level after miR-338-3p overexpression of SCs co-cultured with DRG neurons.
Figure 4|Construction of the miR-221 lentivirus system and miR-338 Tet-on lentivirus system.
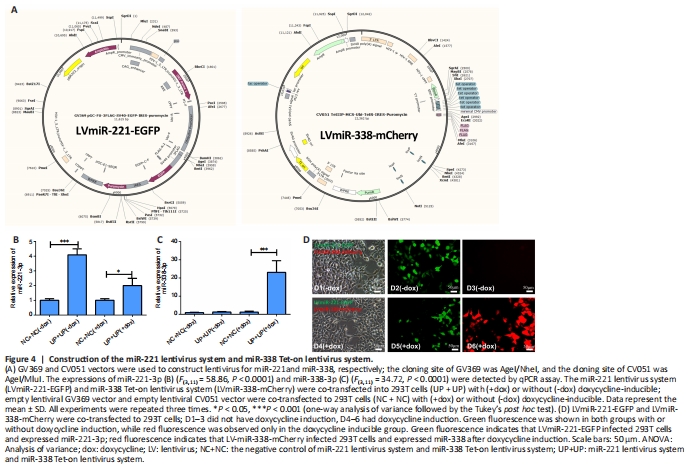
The miR-221 lentivirus system and miR-338 Tet-on lentivirus system can be used together in vivo to sequentially express miR-221-3p and miR-338-3p to regulate the function of SCs after sciatic nerve injury. We next generated miR-221 lentivirus and a doxycycline-inducible miR-338 Tet-on lentivirus system, allowing for control of miR-338-3p overexpression, as described in Methods (Figure 4A). Enhanced green fluorescence protein gene (EGFP) that shows green fluorescence was inserted into LvmiR-221-EGFP and mcherry gene that shows red fluorescence was inserted into LvmiR-338-mCherry. qPCR and immunofluorescence showed that miR-221-3p was expressed in both 293T cell groups with or without doxycycline induction, while miR-338-3p was only expressed in the doxycycline-inducible group (Figure 4B–D). These findings verify that the miR-338 Tet-on lentivirus system can be controlled by doxycycline.
Figure 5|The expression time of miR-221 lentivirus system and miR-338 Tet-on lentivirus system and the formation time of regenerated fiber bridge within nerve conduits in vivo.
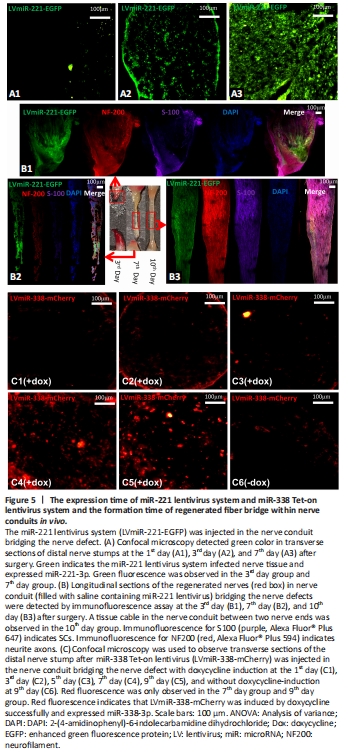
Green fluorescence was observed at the 3rd day in sciatic nerve stumps after LvmiR-221-EGFP was injected into nerve conduits (Figure 5A), which suggests that expression of miR-221-3p took 3 days. At the 3rd day after injection of LvmiR-221-EGFP, we observed a small amount of nerve tissue growing into the nerve conduit from both proximal and distal nerve stumps. At the 7th day, there was a fibrous connection throughout the gap, but it was very thin; at the 10th day, there was an obvious tissue cable in the nerve conduit between the two nerve ends (Figure 5B). NF200 and S100 were weakly expressed in regenerated nerve tissue at the 7th day. S100 fluorescence indicates that SCs have crossed the gap at the 10th day, while NF200 fluorescence indicates that axons grow from the proximal end to the distal end. SCs and axons were arranged orderly at the 10th day. Hence, miR-338 overexpression to promote myelination should be induced at the 10th day after injection of miR-221. Rats injected with LvmiR-338-mCherry, which is induced by doxycycline, expressed miR-338-3p at 7 days after drinking water containing doxycycline (Figure 5C). Therefore, from the results of Figure 5B and C, we deduced that rats should be given doxycycline at the 3rd day after co-injection of LvmiR-221-EGFP and LvmiR-338-mCherry into the nerve conduits in vivo.
Figure 7|Regenerated tissue cable contiguous with the sciatic nerve at the proximal and distal ends at 3 months post-surgery.
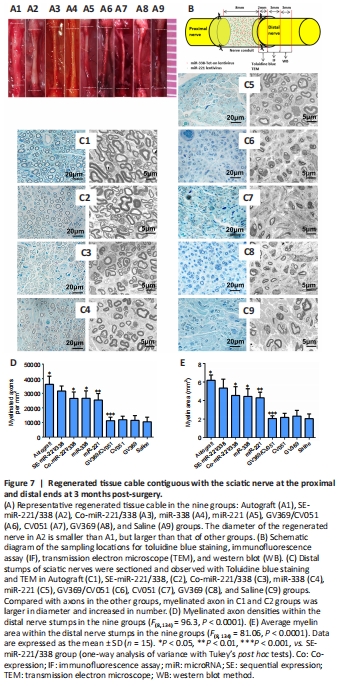
Figure 7A shows regenerated nerves in all groups at 3 months after operation. The diameter of the regenerated nerve in the SE-miR-221/338 group was smaller than that of the autograft group, but larger than those of other groups. A schematic diagram is shown in Figure 7B for the sampling locations for detecting histomorphology and protein expression. Three months after surgery, toluidine blue staining and TEM (Figure 7C) were performed to detect the myelination level of regenerated nerves in distal nerve stumps. Figure 7D shows the density of myelinated axons analyzed from the toluidine blue staining, and Figure 7E shows the area of myelin in each group analyzed from TEM. The highest density of myelinated axons (P = 0.047 autograft vs. SE-miR-221/338) and highest average area (P = 0.027 autograft vs. SE-miR-221/338) of myelin were found in the autograft group followed by the SE-miR-221/338 group; the results were significantly higher than those of other groups. These results indicate that sequential expression of miR-221-3p and miR-338-3p can optimize nerve regeneration through orderly regulation of SC proliferation and myelination.
Figure 8|Expression of marker proteins in regenerated nerves.
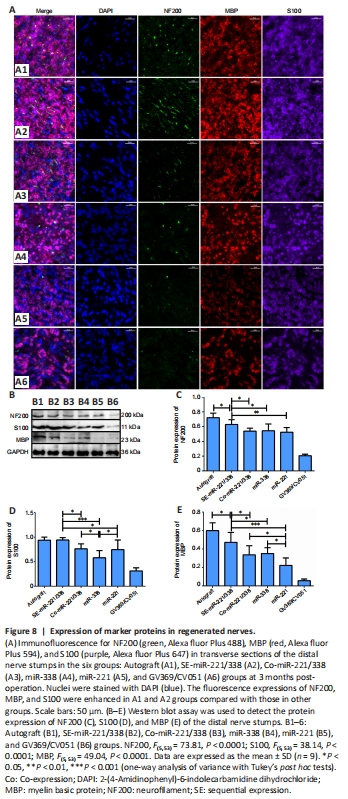
Because there were no statistical differences in SFI, conduction velocities and muscle recovery rate among GV369/CV051, CV051, GV369, and Saline groups (P > 0.05), we used only the GV369/CV051 group as the control group in subsequent analyses. Immunofluorescence and western blot were used to detect the expressions of NF 200, S100, and MBP in regenerated nerves (Figure 8). The fluorescence intensity and protein level of NF200 in the SE-miR-221/338 group (P = 0.028 vs. autograft group, P = 0.030 vs. Co-miR-221/338 group, P = 0.049 vs. miR-338 group, P = 0.007 vs. miR-221) were higher than those of other groups except the autograft group (Figure 8C). There was no significant difference in NF200 protein level among the Co-miR-221/338, miR-338, and miR-221 groups. The fluorescence intensities of S100 in the autograft and SE-miR-221/338 groups were stronger than those of other groups (Figure 8A, B and D). S100 expression showed no difference between SE-miR-221/338 and autograft groups, but it was higher than other groups (P = 0.023 SE-miR-221/338 vs. Co-miR-221/338, P < 0.0001 vs. miR-338, P = 0.01 vs. miR-221). S100 expression in the miR-338 group was lower than that in the Co-miR-221/338 group (P = 0.019) and miR-221 group (P = 0.042). MBP expressions in the SE-miR-221/338 group were higher than those of other groups except the autograft group (P = 0.019 vs. autograft, P = 0.012 vs. Co-miR-221/338, P = 0.029 vs. miR-338, P < 0.0001 vs. miR-221; Figure 8A–C); the lowest protein level of MBP was in the miR-221 group (P = 0.044 Co-miR-221/338 vs. miR-221, P = 0.018 miR-338 vs. miR-221). These results show that the level of nerve regeneration and myelination in the SE-miR-221/338 group was closest to that of the autograft group and was better than that of other groups.
Figure 11|Suppression of Cdkn1b reverses the inhibitory effects of miR-221-3p inhibitor on proliferation and migration of SCs and suppression of Nrp1 reverses the inhibitory effects of miR-338-3p inhibitor on myelination of SCs.
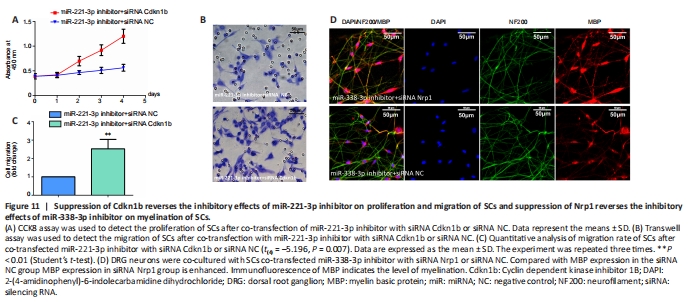
To determine whether Cdkn1b participates in miR-221-3p regulation of proliferation and migration of SCs, miR-221-3p inhibitor was transfected into SCs with siRNA Cdkn1b or siRNA NC. CCK8 and Transwell assays showed that suppression of Cdkn1b significantly promoted proliferation and migration of SCs transfected with miR-221-3p inhibitor (Figure 11A–C).
miR-338-3p inhibitor was transfected into SCs with siRNA Nrp1 or siRNA NC to determine whether Nrp1 participates in miR-221-3p regulation of myelination of SCs. Immunofluorescence assay showed that suppression of Nrp1 by siRNA significantly promoted myelination of SCs transfected with miR-338-3p inhibitor co-cultured with DRG neurons (Figure 11D).
点击此处查看全文