周围神经损伤
-
Figure 1|Enhancement of motoneuron neurite outgrowth by N-Fbs in vitro.

The Transwell co-culture experiments were carried out to investigate the effect of Fbs from two different tissues on motoneuron neurite outgrowth. Following co-culture with motoneurons for 12 hours, N-Fbs showed a trend of promoting neurite outgrowth but without statistical difference compared to C-Fbs (P = 0.4246). Following co-culture for 24 hours, N-Fbs significantly enhanced neurite growth (P = 0.0025) but failed to affect neurite branch number (P = 0.6077). C-Fbs co-cultured with motoneurons failed to promote neurite outgrowth (P = 0.8533; Figure 1).
Figure 2|Effects of N-Fbs or C-Fbs on neurite outgrowth of mononeurons in vitro.
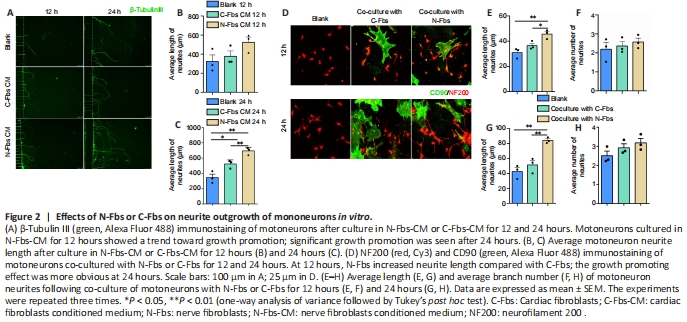
The results from culture of motoneurons in N-Fbs-CM or C-Fbs-CM for 12 hours (P = 0.1244) and 24 hours (P = 0.0012) showed similar results as the Transwell co-culture experiments (Figure 2A–C). The results from co-culture of motoneurons with N-Fbs or C-Fbs for 12 hours (P = 0.0064) and 24 hours (P = 0.0018) indicated that N-Fbs significantly augmented neurite length compared with C-Fbs and the neurites grew along the N-Fbs (Figure 2D–H).
Figure 4|BDNF expression in N-Fbs at injured and normal sciatic nerve sites in rats in vivo.
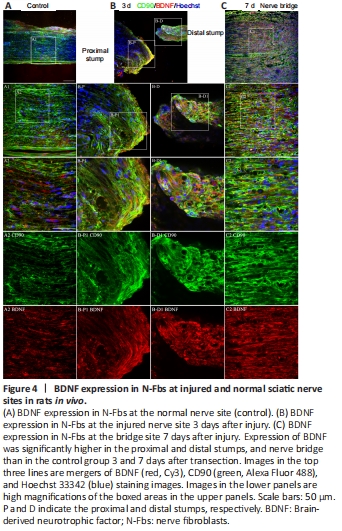
In humans, expression of BDNF and NGF is higher in N-Fbs than in SCs (Weiss et al., 2016). Our mRNA sequencing and qPCR experimental data demonstrated for the first time that Bdnf is highly expressed in rat N-Fbs. To further analyze BDNF expression and localization following peripheral nerve injury, we performed immunohistochemistry analysis at 3 and 7 days after sciatic nerve transection in rats. A previous study reported that Fbs accumulate at the nerve injury site (Parrinello et al., 2010). We found accumulation of a large number of Fbs in the proximal and distal stumps of the injured sciatic nerve 3 days after nerve injury. Furthermore, BDNF expression in Fbs was higher in injured nerves than in normal nerves (Figure 4A and B). BDNF was also expressed in SCs in the proximal and distal stumps 3 days after nerve injury (Additional Figure 3). On day 7 after nerve injury, the proximal and distal stumps were connected by a nerve bridge, a large number of Fbs had accumulated along the bridge, and level of BDNF expression was high at the site (Figure 4C).
Figure 5|BDNF expression in N-Fbs and C-Fbs.
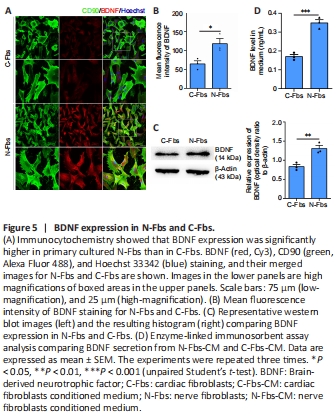
Immunocytochemistry showed that BDNF was mainly located in the cytoplasm and its expression was significantly higher in N-Fbs than in C-Fbs (P = 0.0283; Figure 5A, and B). Western blot analysis provided results consistent with those of immunohistochemistry staining (P = 0.0092; Figure 5C). ELISA showed that the BDNF level in the supernatant released from N-Fbs-CM was significantly higher than that in the supernatant released from C-Fbs (P = 0.0009; Figure 5D).
Figure 6|N-Fbs effects are abolished by TrkB receptor inhibition.
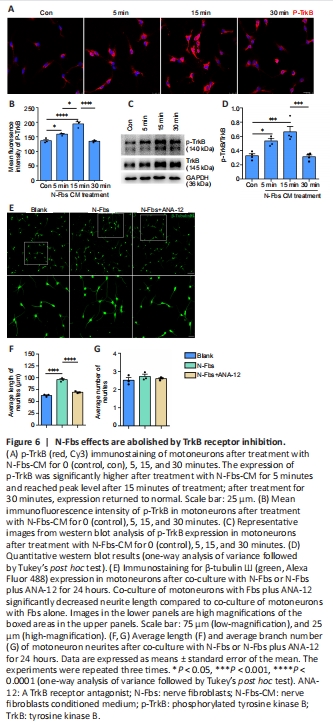
BDNF signaling is elicited through two cell surface receptors: the high affinity ligand-specific tropomyosin receptor kinase B (TrkB), and the low affinity p75 neurotrophin receptor (p75NTR) (McGregor and English, 2019). To determine whether BDNF was produced by N-Fbs through TrkB receptor signaling, motoneurons were treated with N-Fbs-CM (cultured in N-Fbs-CM). Immunostaining showed that p-TrkB expression was significantly higher after treatment with N-Fbs-CM for 5 minutes and reached peak level after 15 minutes of treatment (P < 0.0001; Figure 6A and B). Western blot analysis confirmed the immunostaining results (P = 0.0010; Figure 6C and D).
A Transwell co-culture assay was used to investigate the effect of ANA-12 (a TrkB antagonist) on motoneuron neurite outgrowth. Interestingly, co-culture of motoneurons with Fbs plus ANA-12 significantly decreased neurite length compared to co-culture of motoneurons with Fbs alone (P < 0.0001); however, neurite branch number was not affected (P = 0.7389; Figure 6E–G).
Figure 8|F-actin and β-actin expression is increased by BDNF released from N-Fbs through the ERK and AKT pathways.

Neuronal neurites regenerate through the formation of growth cones, which requires coordination of the cytoskeletal proteins actin and tubulin for axon growth and guidance (Patodia and Raivich, 2012). To determine whether the effects of BDNF on motoneuron neurite outgrowth were mediated via regulation of the actin cytoskeleton, immunostaining for F-actin and β-actin was performed. After co-culture of motoneurons with N-Fbs for 24 or 48 hours, the expression of F-actin and β-actin in motoneurons was significantly higher. Moreover, this increase was inhibited by U0126 and MK2206 (Figure 8A–E, and Additional Figure 4). Western blot analysis confirmed the results of immunostaining (Figure 8H–I).
Growth cones exhibit different shapes and numbers of filopodia and are classified as type I (collapsed), type II (partially collapsed), and type III (fan-shaped) (Lopez-Verrilli et al., 2013). In our study, after co-culture of motoneurons with N-Fbs for 24 and 48 hours, the percentage of type III (fan-shaped) growth cones was significantly higher; moreover, this increase was inhibited by U0126 and MK2206 (Figure 8F, and G).
点击此处查看全文