神经损伤与修复
-
Figure 1|Upregulated expression of Eftud2 in microglia-mediated inflammatory response.
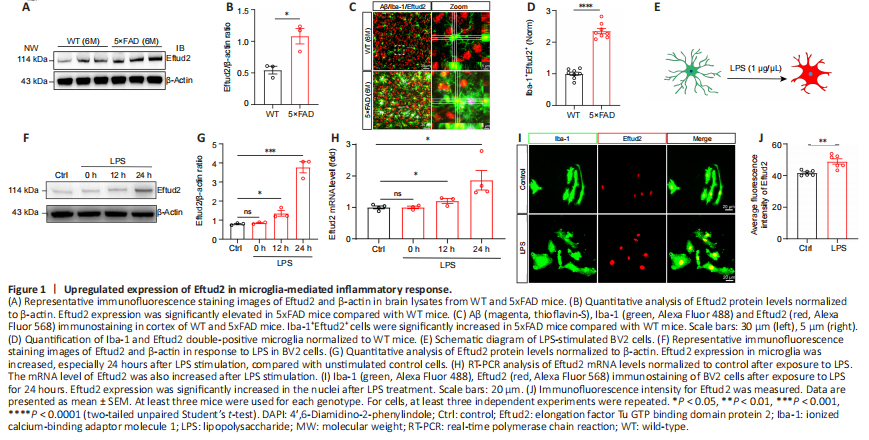
Previous studies have shown that Eftud2 plays an important role in macrophage inflammation (De Arras et al., 2014; Lv et al., 2019). To further determine the potential role of Eftud2 in the inflammatory responses of microglia in the brain, we first examined the expression of Eftud2 in a 5xFAD transgenic mouse model of AD. Immunoblotting indicated that Eftud2 expression in 5xFAD mice was significantly higher than that in control mice (P < 0.05; Figure 1A and B). Given that microglia are significantly activated during AD progression, we next performed immunofluorescence staining on frozen brain sections of wild-type and 5xFAD mice to determine whether the elevated expression of Eftud2 in AD mice was related to microglia (Figure 1C). Immunofluorescence staining showed that the number of Iba-1+Eftud2+ cells in 5xFAD mice was significantly higher than that in control mice (P < 0.0001; Figure 1D). Thus, elevated Eftud2 expression in 5xFAD mice might be due to the activation of microglial cells.
To investigate the role of Eftud2 in microglial activation, LPS was used to stimulate the cultured BV2 microglia at different time points (Figure 1E). Western blot analysis showed that Eftud2 expression in microglia was elevated, especially 24 hours after LPS stimulation, compared with that in unstimulated control cells (12 hours, P < 0.05; 24 hours, P < 0.001; Figure 1F and G). Consistent with these findings, RT-PCR analysis showed that Eftud2 mRNA level was also elevated after LPS stimulation (12 hours, P < 0.05; 24 hours, P < 0.001; Figure 1H). We further confirmed the expression of Eftud2 in microglia by immunofluorescence staining, which also showed significantly elevated immunopositivity of Eftud2 in the nuclei after LPS treatment compared with controls (P < 0.01; Figure 1I and J). Taken together, these results suggest that Eftud2 might be involved in the regulation of microglial activation and inflammatory response in aging and degenerative brain diseases.
Figure 2|Eftud2-deficient microglia exhibit enhanced proliferation.
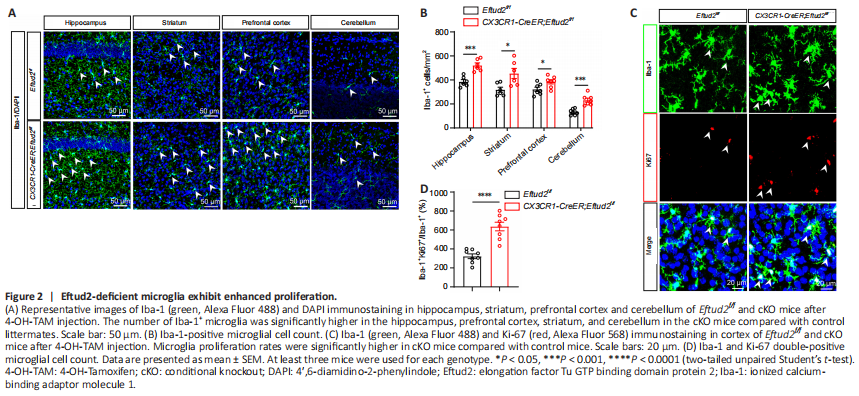
Next, we crossed Eftud2f/f mice (Lv et al., 2019) with CX3CR1-CreER mice expressing tamoxifen-induced Cre recombinase to generate CX3CR1-CreER; Eftud2f/f cKO mice. Furthermore, we injected 4-OH-TAM intraperitoneally at either P14 or P30 to obtain microglia-specific Eftud2 knockout mice. Injection of 4-OH-TAM led to the deletion of Eftud2 in microglia (P < 0.0001; Additional Figure 2C and D), but not in GFAP+ astrocytes or NeuN+ neurons (Additional Figure 2E and F). Then, we used 4-OH-TAM-injected CX3CR1-CreER;
Eftud2f/f mice as cKO mice and Eftud2f/f mice as controls to examine whether the number of microglia varied in different brain regions after ablation of Eftud2 at P30 (Figure 2A). The number of Iba-1+ microglia was significantly higher in the hippocampus, prefrontal cortex, striatum, and cerebellum in the cKO mice compared with that in control littermates (prefrontal cortex, striatum, P < 0.05 or hippocampus, cerebellum, P < 0.001; Figure 2B).
The number of microglia in the brain is normally maintained in a relatively stable range by balancing proliferation and apoptosis (Askew et al., 2017). Because Eftud2 deletion increased the number of microglia, we next investigated whether the increased number of microglia in cKO mice was due to an increased proliferative capacity after loss of Eftud2. To test this hypothesis, we carried out immunofluorescence staining using anti-Ki67 antibody, which has been widely used as a marker of cell proliferation (Sobecki et al., 2016; Remnant et al., 2021). We observed a dramatically elevated number of Ki67+ cells colocalizing with Iba1+ cells in the brain of cKO mice after tamoxifen induction compared with the controls (Figure 2C). To further investigate the effect of Eftud2 deletion on microglia proliferation, we quantified microglia proliferation by counting the number of Ki67+Iba1+ double-positive cells. Microglia proliferation rates were significantly higher in cKO mice than in control mice (P < 0.0001; Figure 2D).
Figure 3|Eftud2-deficient microglia show morphological transformation.
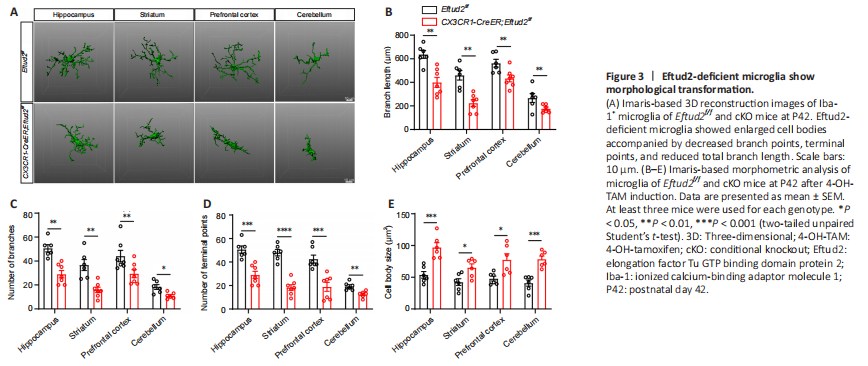
Previous studies have shown that the increase in microglia number is concentrated in the first 3 weeks after birth (Nikodemova et al., 2015; Lv et al., 2019). To examine the effect of Eftud2-specific knockout on developing microglia, 4-OH-TAM treatment was administrated to cKO mice at P14. We found that the number of microglia in different brain regions of cKO mice was significantly elevated compared with that in controls at P21 (striatum, P < 0.05, hippocampus, prefrontal cortex, P < 0.01 or cerebellum, P < 0.001; Additional Figure 3A and B). Accordingly, many more Ki67+Iba1+ double-positive cells were observed in cKO mice than in control littermates (P < 0.0001; Additional Figure 3C and D).
The morphology of microglia is closely related to its function (Perry et al., 2010; Morrison and Filosa, 2013). To better understand the role of Eftud2 in microglia, we next evaluated microglial morphology in different brain regions, including the hippocampus, prefrontal cortex, striatum and cerebellum, using Imaris software (Figure 3A). Eftud2-deficient microglia showed enlarged cell bodies (prefrontal cortex, striatum, P < 0.05 or hippocampus, cerebellum, P < 0.001) accompanied by decreased branch numbers (cerebellum, P < 0.05, hippocampus, prefrontal cortex, striatum, P < 0.001), terminal points (cerebellum, P < 0.01, hippocampus, prefrontal cortex, P < 0.001, striatum, P < 0.0001), and reduced total branch length (hippocampus, striatum, prefrontal cortex, cerebellum, P < 0.01) than in control mice (Figure 3B–E). Consistent with previous results, the morphological changes of microglia were similar across the multiple brain regions (Figure 3A). Moreover, we observed the same morphological changes in Eftud2-depleted developmental microglia at P14, including enlarged cell bodies (prefrontal cortex, P < 0.05; striatum, P < 0.001; hippocampus and cerebellum, P < 0.0001), decreased branch numbers (hippocampus, prefrontal cortex, and cerebellum, P < 0.001; striatum, P < 0.0001), terminal points (hippocampus, prefrontal cortex, and cerebellum, P < 0.001; striatum, P < 0.0001), and reduced total branch length (striatum, P < 0.01; hippocampus, prefrontal cortex, and cerebellum, P < 0.001) than in control mice (Additional Figure 4A–E). These results further suggest that despite the heterogeneity of microglia in different brain regions, Eftud2 may have a common function in microglia throughout the brain. Taken together, our results suggest that Eftud2 deletion in microglia leads to dysregulation of cell proliferation and morphological transformation with amoeboid cell body and short branches.
Figure 4|Eftud2 deficiency promotes activation of anti-inflammatory phenotype microglia.
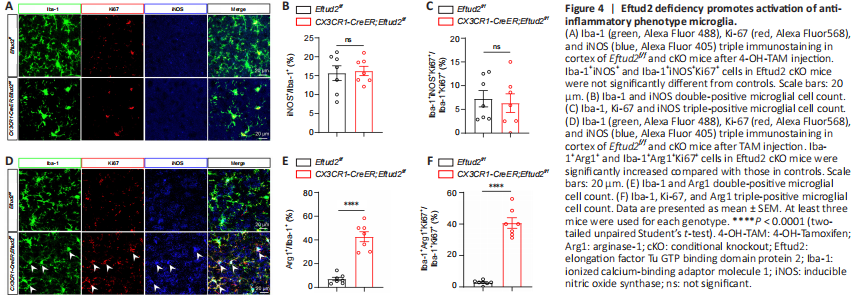
Activated microglia are typically divided into two states: M1 (also known as classically activated) with a proinflammatory phenotype, and M2 (known as alternatively activated) with an anti-inflammatory phenotype (Cherry et al., 2014; Ronaldson and Davis, 2020). Therefore, we used iNOS and Arg1, markers of the proinflammatory and anti-inflammatory phenotypes, respectively (Tang and Le, 2016), costained with Iba-1 to determine the subtype of activated microglia (Figure 4A–D). Eftud2 cKO mice had a significantly elevated number of Iba-1+Arg1+ cells (P < 0.0001), but not Iba-1+iNOS+ cells (P > 0.05), compared with controls (Figure 4B–E). These results indicated that Eftud2 ablation induced the activation and transformation of microglia into the anti-inflammatory phenotype instead of the proinflammatory phenotype. To further identify the phenotype of the increased population of Ki67+Iba1+ microglial cells in Eftud2 cKO mice, iNOS and Arg1 were costained with both Iba1 and Ki67 (Figure 4A–D). Ki67+Iba1+ staining was mainly present in Arg1+ cells (Ki67+Iba1+Arg1+, P < 0.0001), whereas there was little or no expression in iNOS+ cells (Ki67+Iba1+iNOS+, P > 0.05; Figure 4C–F). Taken together, our data indicated that Eftud2 deficiency specifically enhanced the proliferation of the anti-inflammatory phenotype microglia.
Figure 5|Eftud2 knockdown promotes anti-inflammatory phenotype activation in BV2 cells
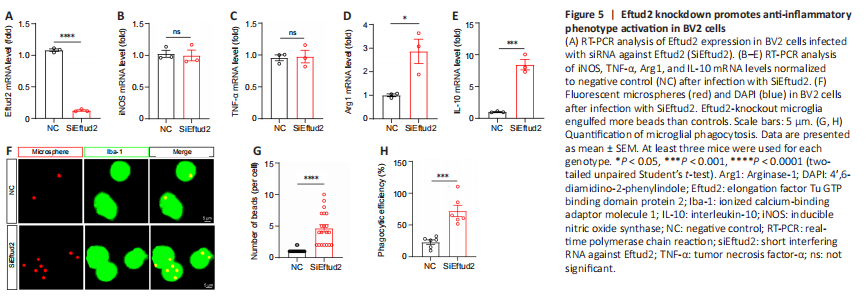
In addition, we knocked down Eftud2 in BV2 microglia with siRNA specifically targeting Eftud2 (P < 0.0001, compared with controls; Figure 5A). Transcription of inflammatory cytokines and microglia phenotypic markers were detected by RT-PCR in control and Eftud2 knockdown BV2 cells. Compared with the control, Eftud2 knockdown did not significantly alter the expression of iNOS and TNFα (P > 0.05; Figure 5B and C). In contrast, the mRNA levels of Arg1 and IL-10 were significantly upregulated in Eftud2 knockdown microglia compared with controls (P < 0.05 and P < 0.001, respectively; Figure 5D and E). We also assessed the phagocytic activity of microglia after Eftud2 knockdown by fluorescent microspheres (Figure 5F). Eftud2 knockdown microglia engulfed more beads than control microglia (number of beads, P < 0.0001 or phagocytic efficiency, P < 0.001; Figure 5G and H). This indicated that Eftud2 knockdown microglia had stronger phagocytic activity. Taken together, our data suggested that Eftud2 deletion promoted the activation of anti-inflammatory phenotype microglia.
Figure 6|Eftud2 regulates proinflammatory and anti-inflammatory phenotype activation of microglia involved in inflammatory responses via the NF-κB pathway.
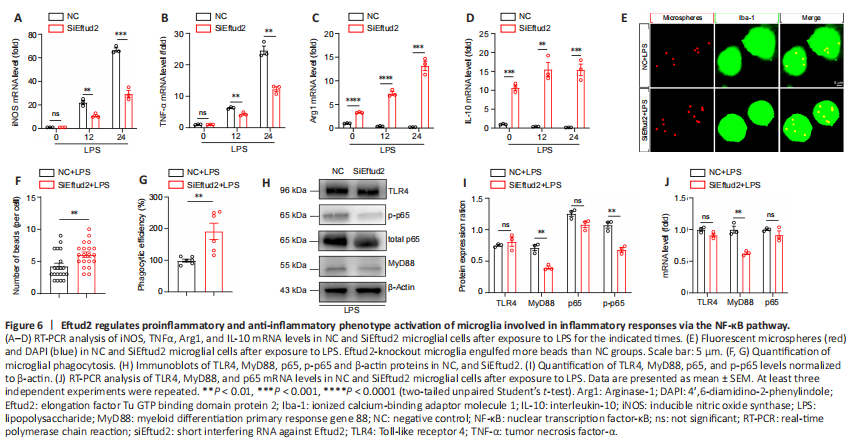
Many studies have demonstrated that M2 phenotype activation of microglia is protective (Ahmed et al., 2017; Ji et al., 2019). Given that Eftud2 deficiency promoted anti-inflammatory phenotype activation of microglia, we speculated that Eftud2 deletion in microglia may suppress inflammatory responses by promoting anti-inflammatory phenotype activation. To investigate this, we used LPS to stimulate BV2 microglia for 0, 12, and 24 hours, respectively. RT-PCR analysis showed that the expression of iNOS and TNFα in Eftud2 knockdown microglia was significantly suppressed after LPS stimulation, compared with control (12 hours, P < 0.01 or 24 hours, P < 0.001; Figure 6A and B). Moreover, we also found that knockdown of Eftud2 significantly increased the expression levels of Arg1 (24 hours, P < 0.001 or 0, 12 hours, P < 0.0001) and IL-10 under LPS stimulation (12 hours, P < 0.01, 12 hours, 24 hours, P < 0.001; Figure 6C and D). In addition, we observed that LPS-stimulated macrophage phagocytosis of fluorescent microspheres was significantly enhanced in Eftud2-knockdown BV2 microglial cells, which had a higher number of fluorescent microspheres within the soma when compared with controls (P < 0.01; Figure 6E–G). This suggested that Eftud2 may be involved in LPS-induced inflammatory responses by regulating the activation of proinflammatory and anti-inflammatory phenotypes.
Next, we investigated the possible signaling mechanism by which Eftud2 regulates microglial activation in the inflammatory response. The TLR4/NF-κB pathway has been shown to be involved in polarization of the microglia M1/M2 phenotypes (Zhang et al., 2019). Previous studies have also shown that LPS activates NF-κB signaling (Bode et al., 2012; Zhou et al., 2020). To determine whether the TLR4/NF-κB signaling pathway was affected in Eftud2-deficient BV2 cells, we used western blot assay to detect the expression of several important NF-κB signalsomes, including TLR4, MyD88, and total and p-p65, in Eftud2-deficient and control cell lysates. Eftud2 knockdown significantly decreased the expression of MyD88 and p-p65 compared with control cells (P < 0.01), and the expression of total p65 and TLR4 was not affected in Eftud2 deficient cells (P > 0.05; Figure 6H and I). Notably, we also found downregulation of MyD88 (P < 0.01) and no significant changes in TLR4 and p65 (P > 0.05) at the mRNA level (Figure 6J). Taken together, these results indicated that suppression of NF-κB signaling pathway activation is involved in Eftud2 deficiency-induced phenotypic activation of microglia in inflammatory responses.