神经损伤与修复
-
Figure 2|FN promotes the proliferation of NG2+ cells.
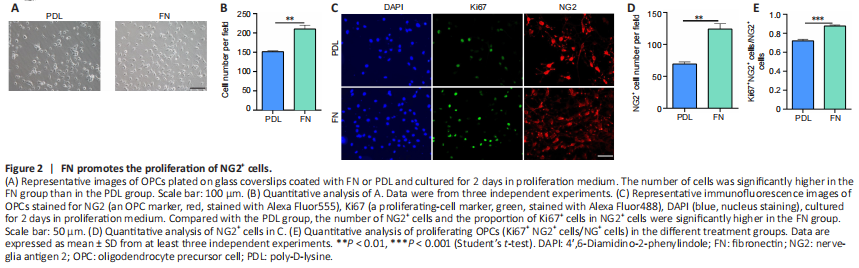
In the first experiment, we studied how FN affects OPC proliferation. Cells were isolated from P0–P2 mice and purified to over 98% OPC purity. Our data showed that the percentage of OLs did not depend on the coating substance (PDL, FN, PLO, PLO + FN) (Additional Figure 1A–C). However, the total number of cells was significantly greater in FN-coated plates than in PDL-coated plates (Figure 2A and B). Immunofluorescence staining showed that the number of OPCs (NG2+ cells) was significantly greater in FN-coated plates than in the PDL-coated plates (Figure 2C and D). Furthermore, the percentage of proliferating OPCs (Ki67+ NG2+ cells/NG2+ cells) was significantly higher in FN-coated plates than in PDL-coated plates (Figure 2C and E). These results indicated that FN promotes OPC proliferation.
Figure 3|FN suppresses the differentiation of oligodendrocytes.
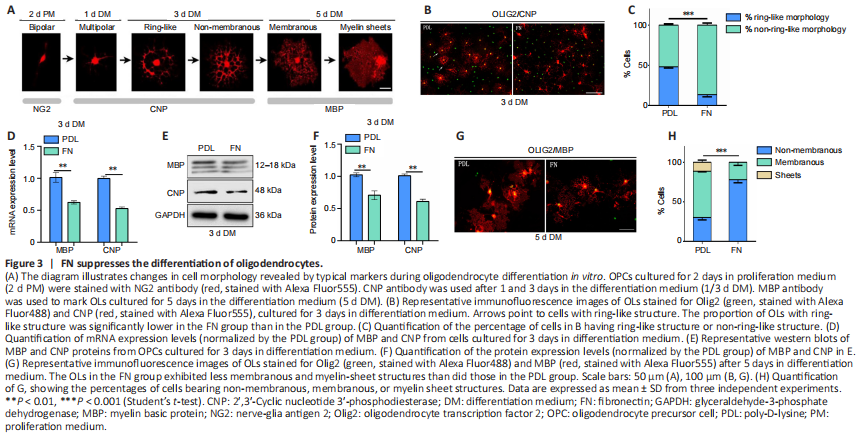
Cultured OPCs can be induced to differentiate in vitro, following a similar time course as occurs in vivo (Pfeiffer et al., 1993; Warrington et al., 1993; Miller, 2002; Emery, 2010). During this process, the cell morphology undergoes a series of changes. In the initial stage, cell shape changes from mostly bipolar to multi-process. Next, cell processes connect to form a ring-like structure, and then a double-ring structure. Then, they fuse into myelin membranes which change into flat myelin sheets (Bradl and Lassmann, 2010; Tiane et al., 2019; Figure 3A). During this in vitro differentiation process, the cells express the same markers with the same timing as they do in vivo (Figure 3A). After 1 day in differentiation medium, the number of CNP+ (a marker for early OPC differentiation; Fressinaud and Eyer, 2013) cells appeared fewer and cell processes were less elaborate among OPCs cultured on FN-coated plates than among those cultured on PDL-coated plates (Additional Figure 2A). The fractal dimension value (D) was used to measure the complexity of cell morphology. We found that the D value was significantly lower for the FN group than for the PDL group after one day of differentiation (Additional Figure 2B and C). After 3 days of differentiation, while many cells in the PDL group exhibited ring-like structures, very few did so in the FN group, indicating more advanced differentiation in the PDL group (Figure 3B and C). We also measured the area of MBP+ staining, the fluorescence intensity of the signal, and the corrected total cell fluorescence. Statistical analysis showed that these parameters were significantly lower in the FN group than in the PDL group (Additional Figure 2C and D), indicating a less differentiated morphology. Consistent with these differences in cell morphology, MBP and CNP mRNA and protein levels were significantly lower in the FN group than in the PDL group (Figure 3D–F). After 5 days of differentiation, the cells in the FN group continued to be less differentiated than cells in the PDL group, with less myelin membranous/sheets structure (Figure 3G and H), a smaller area of MBP+ staining, and lower fluorescence intensity of the signal and corrected total cell fluorescence (Additional Figure 2E and F). These data indicated that FN suppresses OL differentiation.
Figure 4|PLO blocks the negative effects of FN on oligodendrocyte differentiation and promotes oligodendrocyte maturation.
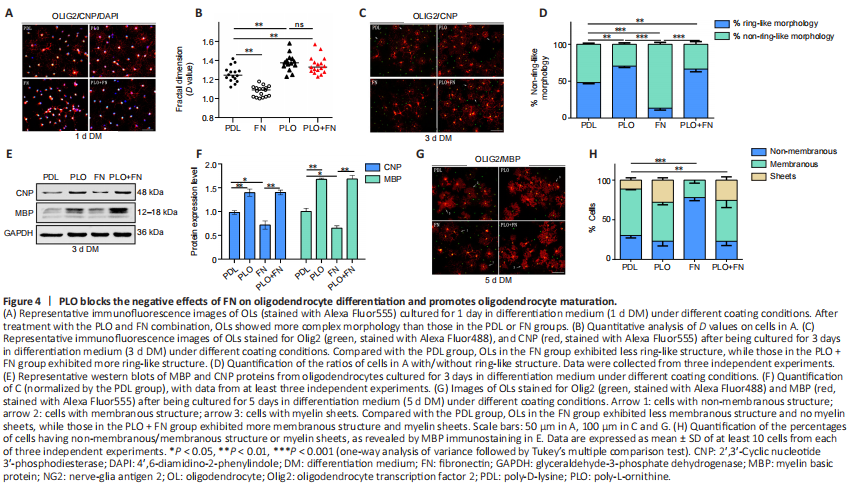
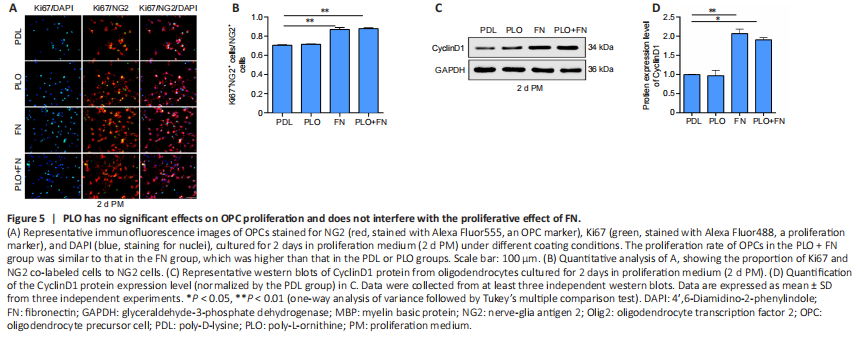
Figure 5|PLO has no significant effects on OPC proliferation and does not interfere with the proliferative effect of FN.
PLO is commonly used in neuronal stem cell culture (Sato et al., 2018; Hosseini Farahabadi et al., 2020) because it can promote the differentiation of neuronal stem cells (Ge et al., 2015). In the next experiment, we tested the effect of PLO on OL differentiation from OPCs in culture. After one day in the differentiation medium, cells in the PLO group exhibited more processes than those in the PDL group. Additionally, the D value was significantly higher for the PLO group than for the PDL group (Figure 4A and B). After 3 days of differentiation, more CNP+ OLs with ring-like structures appeared in the PLO group than in the PDL group (Figure 4C and D). Furthermore, CNP and MBP protein levels were much higher in the PLO group (Figure 4E and F). After 5 days of differentiation, OLs in PLO group showed mature morphology with a higher percentage of cells with membranous structures and myelin-sheet structures than was found in the PDL group (Figure 4G and H). These results indicated that PLO promotes OL differentiation.
Next we tested whether PLO could block the inhibitory effect of FN on OL differentiation. We coated the culture plates with both FN and PLO (PLO + FN). On the first day of differentiation, OLs in the PLO + FN group exhibited many more processes and higher D values than did the cells in the FN group (Figure 4A and B). On the third day, analysis showed that the proportion of cells with ring-like structures had risen and was significantly greater in the PLO + FN group (PLO + FN vs. FN) (Figure 4C and D). Similarly, CNP and MBP protein levels were also significantly higher in the PLO + FN group (PLO + FN vs. FN) (Figure 4E and F). On the fifth day, cells in the PLO + FN group showed mature morphology with membranous and myelin structures visible in almost all cells (Figure 4G). Statistical analysis showed that on this day, the proportion of cells with membranous and myelin structures was significantly higher in the PLO + FN group than in FN group, while the proportion of cells with non-membranous structures was drastically lower (Figure 4H). At each time point tested, signs of OL differentiation were greater in the PLO + FN group than in the FN group. The degree of differentiation seen in the FN + PLO group almost reached what was observed in the PLO group (Figure 4). These results indicated that PLO abolished the inhibitory effects of FN on OL differentiation.We also tested the influence of PLO on OPC proliferation, finding that it did not alter the amount of proliferation (PDL vs. PLO, P > 0.05). Furthermore, FN-induced increases in OPC proliferation also remained unchanged by the addition of PLO (FN vs. FN + PLO, P > 0.05) (Figure 5A and B). The levels of cyclinD1 exhibited a similar pattern, being equally higher in FN and PLO + FN groups than in the PLO group (Figure 5C and D). These results suggest that PLO does not interfere with the FN-induced OPC proliferation.
Figure 7|PLO promotes functional myelin repair after 14 days of lysolecithin-induced demyelination injury.
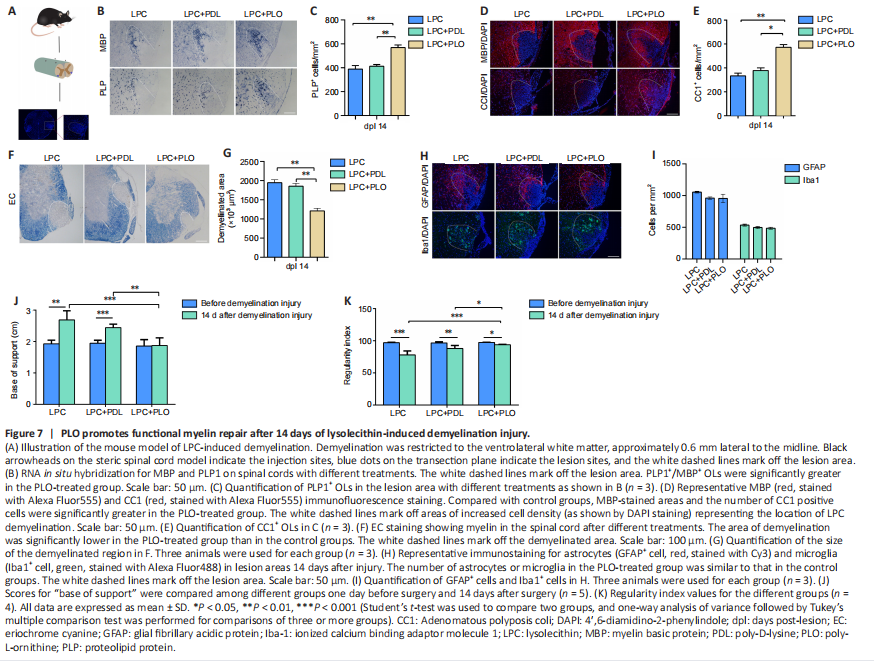
Thus far, we have shown that in culture, PLO can block FN-regulated inhibition of OL differentiation while sparing OPC proliferation. To evaluate the practical potential of PLO as a therapeutic agent for diseases of demyelination, we used an animal model of LPC-induced demyelination. Mouse groups mirrored the groups used in the culture studies. Local injection of LPC into the spinal cord causes selective and focal myelin loss in the ventrolateral white matter (Torre-Fuentes et al., 2020; Figure 7A). Fourteen days after injection, which is during active remyelination (Keough et al., 2015), the injured segments were serially sectioned on a cryostat for analysis. The differentiated OLs were detected by in situ hybridization for proteolipid protein (PLP1) and myelin basic protein (MBP) mRNAs. As shown in Figure 7B and C, the number of differentiated OLs was significantly higher in the PLO-treated group than in controls (LPC and LPC + PDL). These results were confirmed by immunostaining with CC1 and MBP antibodies, showing a higher density of CC1+ mature OLs and a significantly enlarged area of MBP protein expression after PLO treatment (Figure 7D and E). We also used eriochrome cyanine staining for myelin, which revealed that the area of demyelination was significantly lower following PLO treatment than after the control treatments (Figure 7F and G). We examined astrocyte and microglia in the damaged region 14 days after injury and found no significant differences between groups in the numbers of Iba1+ microglia/macrophages or GFAP+ astrocytes (Figure 7H and I).
We next investigated the functional recovery of the modal mice using the Catwalk system. Demyelination of the spinal cord in the lower thoracic region affects hindlimb function, as the descending and ascending fibers play important roles in initiating and coordinating locomotion. The mice in control groups (LPC and LPC + PDL) exhibited chronic hindlimb paresis over the treatment period, with a significantly wider base of support between the hind paws. In contrast, animals treated with PLO exhibited a significant improvement in motor function, regaining the use of both hind limbs after 14 days (Figure 7J). We also assessed recovery using the regularity index, a fractional measure of inter-paw coordination (Garrick et al., 2021). This index was significantly higher in PLO-treated mice than in control mice (Figure 7K), indicating better coordination. Overall, our results demonstrated that PLO-treated animals exhibited expedited functional recovery, suggesting that PLO promotes functional recovery of LPC-induced demyelinating injury.
Figure 8|PLO exhibits dose-dependent effects in the animal model of demyelination.
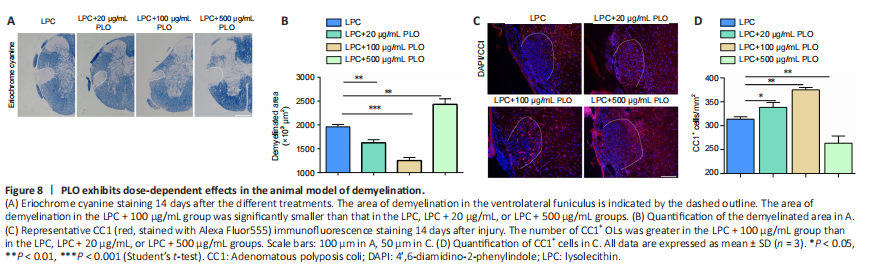
We next explored how dosage affects PLO-facilitated myelin repair. At a low concentration (20 μg/mL), PLO helped to reduce the size of the demyelinated area (vs. controls). At 100 μg/mL, PLO exhibited a much better effect on promoting myelin repair. Compared with controls, at this dosage, the area of myelin damage was greatly reduced and the number of CC1+ OLs was significantly higher in the damaged area (Figure 8A–D). However, at the high PLO concentration of 500 μg/mL, the effect was the opposite; the size of the damaged area was larger and CC1+ cell density was lower (Figure 8A–D) compared with the control. Thus, our data suggests that high levels of PLO will worsen myelin repair and wound recovery.