周围神经损伤
-
Figure 2|Effect of the RPNI on the inhibition of the proliferation of collagenous fibers in the PNS.
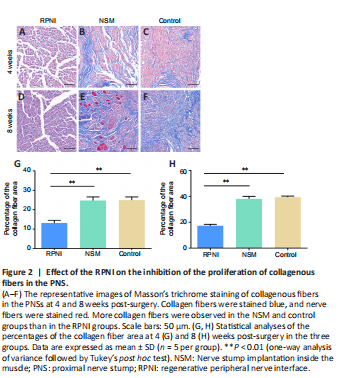
Masson’s trichrome staining showed blue-stained collagenous fibers in the neuromas. At 4 weeks post-surgery, there was a large number of irregular and haphazard blue-stained collagenous fibers in the control and NSM groups. Muscle tissues also grew into the PNS in the NSM group. Furthermore, the collagenous fibers gradually proliferated, gathered together, and wrapped around the nerve fibers over time in both the control and NSM groups. In the NSM group, more muscle tissues invaded the nerve stump at 8 weeks post-surgery than at 4 weeks post-surgery. In contrast, no obvious collagenous fibers or muscle tissues were observed in the RPNI group at 4 or 8 weeks post-surgery (Figure 2).
Figure 3|Effect of the RPNI on the inhibition of myofibroblast activity in the PNS.
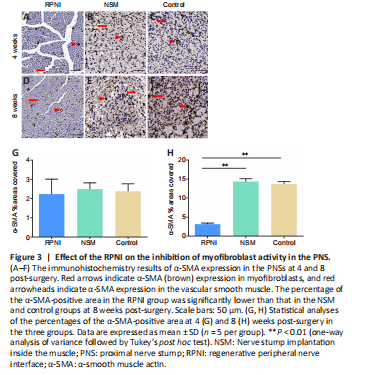
The fibrosis marker α-SMA in the PNS was evaluated using immunohistochemistry staining. At 4 weeks post-surgery, there were no significant differences in the percentage of the α-SMA-positive area in the PNS among the three groups (all P > 0.05). However, the percentage of the α-SMA-positive area in the control and NSM groups increased significantly at 8 weeks post-surgery, and was significantly higher than that in the RPNI group (both P < 0.01; Figure 3).
Figure 4|Effect of the RPNI on the inhibition of the proliferation of irregularly regenerated axons in the PNS.
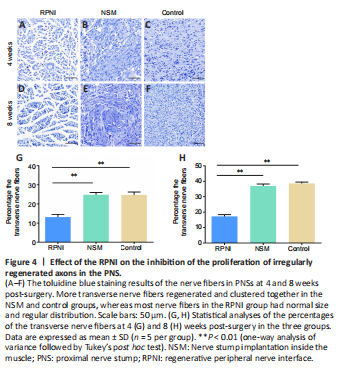
Toluidine blue staining results showed that the regenerated nerve fibers (stained in blue) were densely and chaotically distributed in the NSM and control groups at 4 weeks post-surgery, whereas those in the RPNI group maintained clear and regular morphology. At 8 weeks after axotomy, further regeneration of axons occurred, including many transverse and longitudinal nerve fibers, many of which were clustered together in the NSM and control groups. In contrast, most nerve fibers in the RPNI group were normally sized, regularly distributed, and arranged in an insulated pattern. Furthermore, only a small number of transverse regenerated axons were observed in the RPNI group (Figure 4). Altogether, these findings suggested that RPNI surgery inhibits the accumulation of collagenous fibers and the disordered sprouting of axons at the injury site following neurectomy.
Figure 5|Effect of the RPNI on the inhibition of neuroinflammation in the PNS.
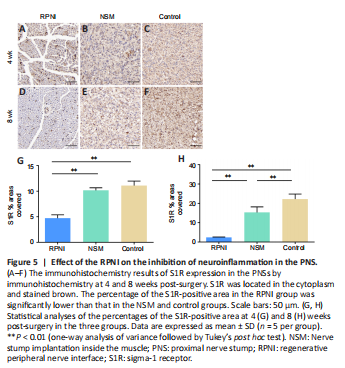
To evaluate the neuroinflammation of the PNS in each group, we examined S1R expression using immunohistochemistry staining. Histomorphological analysis showed that the percentage of the S1R-positive area in the RPNI group was significantly lower than that in the NSM and control groups at 4 and 8 weeks post-surgery (all P < 0.01). In contrast, the percentage of the S1R-positive area in the NSM group was significantly lower than that in the control group at 8 weeks only (P < 0.01; Figure 5). Collectively, this suggested that RPNI surgery prevents neuroinflammation following nerve transection and offers a better anti-inflammatory effect than NSM surgery.