视神经损伤
-
Figure 2|Residual RGCs and axons had survived at 4 weeks after ONC.
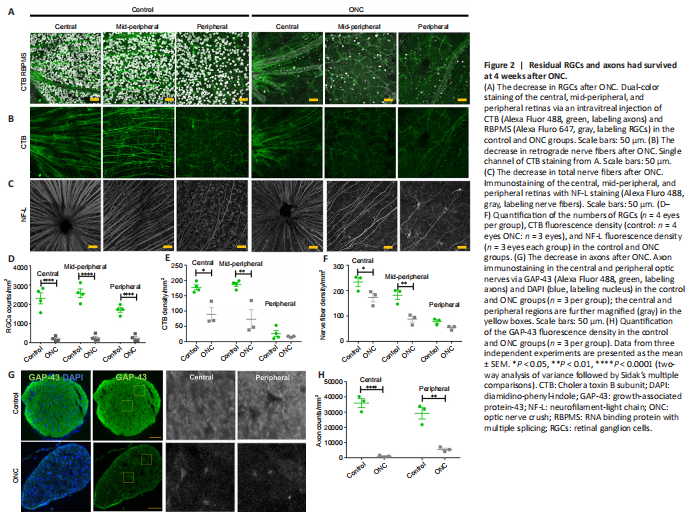
Previous studies have reported that approximately 10% of RGCs had survived at 4 weeks after ONC (Tran et al., 2019). We wanted to determine whether the axons stretching from the RGCs had survived, so we used a pan-RGC marker antibody and an axon marker antibody to stain the RGC and RGC nerve fibers, as well as the axons, after ONC administration in 1 eye of 3-month-old mice. As expected, we found that ONC led to a marked reduction in the number of RGCs (n = 4 eyes per group, Figure 2A upper panel). When compared with the number of RGCs in controls (2314.5 ± 287.9/mm2), the number of RGCs in the central retina 4 weeks after ONC was only 196.0 ± 61.6/mm2. Furthermore, 4 weeks after ONC, the numbers of RGCs in the mid-peripheral retina and peripheral retina were 248.0 ± 85.6/mm2 and 237.0 ± 86.1/mm2, respectively. The survival rates were 8.47%, 9.6%, and 13.9% in the central retina, mid-peripheral retina, and peripheral retina, respectively. To confirm the existence of retrograde nerve fibers, we used the retrograde tracer CTB (Figure 2B lower panel). The results showed that the nerve fibers that remained after ONC included retrograde nerve fibers (control: n = 4 eyes, ONC: n = 3 eyes). Additionally, using an NF-L antibody, we found that 4 weeks after ONC, 74.0%, 48.3%, and 68.3% of the fluorescent signal remained in the central, mid-peripheral, and peripheral retina, respectively (n = 3 eyes per group, Figure 2C). The number of RGCs was significantly reduced (P < 0.0001 for all regions; Figure 2D). The fluorescent signals of CTB-stained nerve fibers were also significantly reduced in the center (P = 0.0019) and mid-peripheral (P = 0.002) retina, respectively (Figure 2E). CTB-stained nerve fibers were less injured in the peripheral retina, and the total numbers of nerve fibers in the central (P = 0.02) and mid-peripheral (P = 0.001) retina 4 weeks after ONC were significantly lower than those in controls (Figure 2F). These data strongly suggest that the nerve fibers in the peripheral retina were less damaged.
We used the axon antibody GAP-43 to stain the axons in the optic nerve (n = 3 per group), and found that the fluorescent signals of the axons were significantly reduced after ONC (center: P < 0.0001 and peripheral: 0.0003, respectively) (Figure 2G and H). However, some axons had survived at 4 weeks after ONC. Collectively, these findings show that RGCs and axons survived at 4 weeks after ONC, including retrograde axons.
Figure 3|Retrograde labeling of RGCs in ONC eyes.
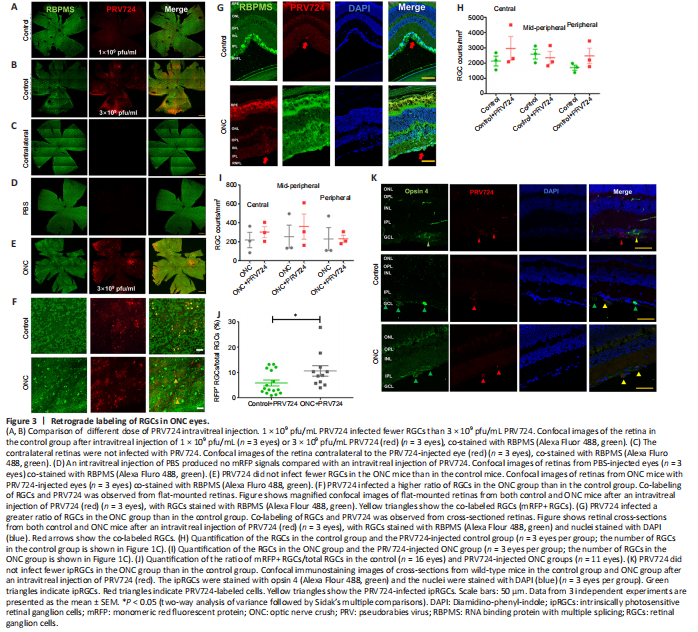
To investigate the retinorecipient regions connected to RGCs, we used retrograde trans-synaptic virus PRV724 constructed with mRFP, an isogenic version of PRV-Bartha. Three weeks after ONC, PRV724 was intravitreally injected into the ONC eyes of the experimental group or the normal eyes of the control group (D21). After 6–7 days (D27–28), we collected whole-mount retinas or frozen retina sections (Figure 1B). Because 1 × 109 pfu/mL PRV724 did not obviously infect RGCs in the control group (n = 3 eyes; Figure 3A), we used 3 × 109 pfu/mL PRV724 in subsequent studies (Figure 3B). There were no red fluorescent signals in the retinas of the contralateral eye (n = 4 eyes; Figure 3C), which was similar to eyes intravitreally injected with PBS (n = 3 eyes; Figure 3D).
To verify the infection specificity of PRV724 in RGCs, we used the pan-RGC marker RBPMS antibody to stain the retina. The results showed that PRV724-labeled RGCs were present in both the control and ONC groups (Figure 3B, E, and F), but the retinas of PRV724-injected eyes were distorted and swollen because of PRV toxicity (n = 3 eyes per group; Figure 3G). To determine whether PRV724 reduced the number of RGCs, we counted the number of RGCs in the control and ONC groups using whole-mount retina staining with RBMPS antibody, which is a pan-RGC marker. When compared with the data in Figure 1D, there was no significant loss of RGCs after intravitreal injection of PRV724 over 5–6 days in the control or ONC groups (n = 3 eyes per group, all P > 0.05; Figure 3H, and I). Nevertheless, the results showed that the PRV724 infection was mostly limited in RGCs regardless of ONC administration. Although there were fewer RGCs in ONC eyes, the proportion of infected RGCs appeared to be higher. By counting the number of RGCs with red fluorescence (mRFP+ RGCs) and the total number of RGCs in the infected regions of retinas, we found that the percentage of mRFP+ RGCs/total RGCs in control eyes following a PRV724 intravitreal injection was 5.8 ± 1.2% (n = 16 eyes), while the percentage of mRFP+ RGCs/total RGCs in ONC eyes was 10.9 ± 2.1% (n = 11 eyes). There was a small but significant difference between these 2 groups (P = 0.04, Figure 3J).
A previous study showed that PRV-Bartha exclusively infects ipRGCs (Viney et al., 2007), and among the 46 types of RGCs, 2 types of αRGCs and all 5 types of ipRGC were injury-resistant to ONC (Rheaume et al., 2018; Tran et al., 2019; Yang et al., 2020). ipRGCs accounted for over 82% of the surviving RGCs 60 days after the ONC procedure (Pérez de Sevilla Müller et al., 2014). Hence, we next confirmed whether PRV724 exclusively infected ipRGCs, as reported in a previous study (Viney et al., 2007). We stained the retinal cross-sections with the ipRGC marker opsin 4 antibody, and found that while some PRV724-infected RGCs were stained with opsin 4, other opsin 4-labeled RGCs were not infected with PRV724 in the control group (n = 3 eyes; Figure 3K upper row). PRV724-infected RGCs were also co-stained with opsin 4 in the ONC group (n = 3 eyes; Figure 3K bottom row). However, not all PRV724-labeled cells were co-stained with opsin 4 (Figure 3K middle row). Hence, PRV724 might not exclusively infect ipRGCs. Additionally, when GAP-43 antibody was used to stain the intact optic nerve crossover at the optic chiasm, no red fluorescent signals were observed (n = 3; Additional Figure 2A–D). This suggests that PRV724 did not reveal the axons of RGCs. Taken together, these findings confirm that PRV724 can label RGCs in ONC eyes.
Figure 4|Overview of tissue-cleared brain.
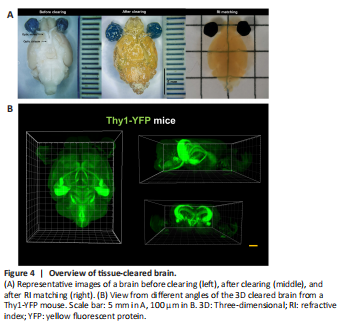
After confirming our labeling strategy, we sought to better identify the whole brain neurons innervated by the RGCs. Accordingly, 6–7 days after the intravitreal injection of PRV724 in the left eye, we removed the whole brain with or without the eyeballs and optic nerve (Thy1-YFP control mice n = 4, wild-type control mice n = 4). The mouse brains were analyzed using whole brain optical tissue clearing, with a transparency time of 7 days (Figure 1C). As expected, the brains were fully transparent with no visible shrinkage (Figure 4A), and they could be imaged via a light-sheet microscope (Figure 4B and Additional Video 1). The locations of different brain areas were determined following standard procedures, as described in the Methods section (Additional Figure 1A–H). To better locate the positions, we used Thy1-YFP mice. We found that PRV724 was able to label the RGCs in C57BL/6 (wild-type) mice as well as in Thy1-YFP mice, regardless of ONC administration (Additional Figure 3A). There was no significant difference in the number of RGCs in the C57BL/6 mice (wild-type) versus Thy1-YFP mice, regardless of ONC administration (n = 3 eyes per group; Additional Figure 3B). Also, the percentage of RFP+RGCs/total RGCs in the C57BL/6 mice (wild-type) and Thy1-YFP mice did not differ significantly, regardless of ONC administration (n = 5 eyes per group; Additional Figure 3C).
Figure 5|Overview of PRV724-traced retinorecipient regions in control and ONC mice.
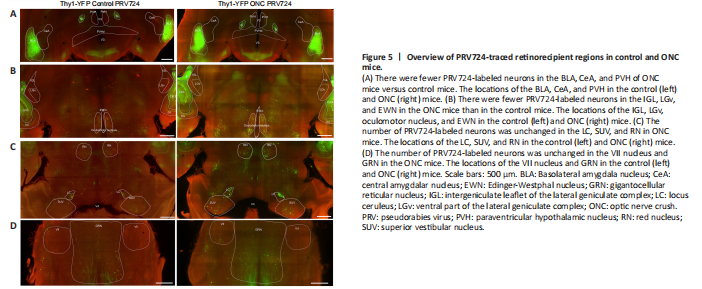
We focused on retinorecipient nuclei known to receive robust RGC innervation including the suprachiasmatic nucleus (SCN), ventral part of the lateral geniculate complex (LGv), intergeniculate leaflet (IGL), olivary pretectal nucleus (OPN), and superior colliculus (SC) (Hattar et al., 2002, 2006; Beier et al., 2021). However, PRV724 labeling of neurons was inconsistent in the SCN, OPN, and SC (data not shown), apart from the LGv and IGL. Hence, data from the SCN, OPN, and SC were not included in this study. Consistent with previous studies using PRV-Bartha (Card et al., 1991; Smith et al., 2000; Pickard et al., 2002; Smeraski et al., 2004), we observed trans-synaptically labeled neurons in the paraventricular hypothalamic nucleus (PVH), Edinger-Westphal nucleus (EWN), oculomotor nucleus, IGL, LGv, and superior vestibular nucleus (SUV) in both hemispheres (Figure 5A–C). Unexpectedly, PRV724 labeled neurons were also observed in the basolateral amygdala nucleus (BLA), central amygdalar nucleus (CeA), red nucleus (RN), VII nucleus, and gigantocellular reticular nucleus (GRN) in both hemispheres (Figures 5A, C, and D).
Figure 6|ONC administration selectively eliminates projections from RGCs in the BLA, CeA, PVH, IGL, LGv, oculomotor nucleus, and EWN.
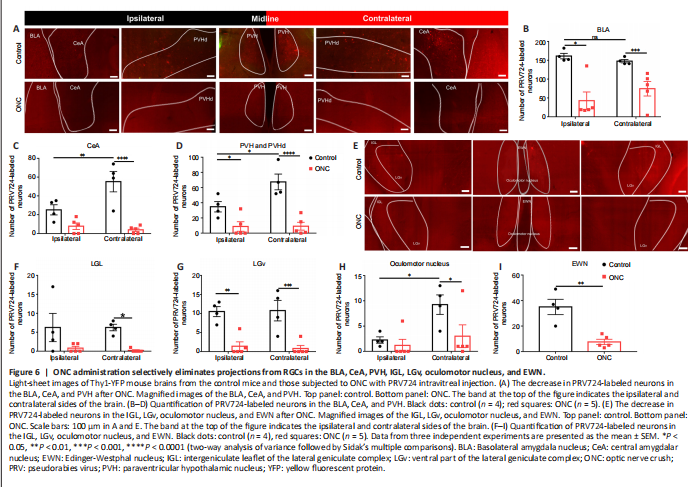
As shown in Figure 6A, the signals of PRV724-labeled neurons were distributed equally across the ipsilateral and contralateral BLA (P = 8498), while the signals of PRV724-labeled neurons were mostly located in the contralateral CeA (P = 0.0073) and contralateral PVH (P = 0.0164) (n = 4; Figure 6B–D). As shown in Figure 6E, PRV724-labeled neurons in the IGL and LGv were distributed equally across both hemispheres (P > 0.9999 and P = 0.9928), while the signals of PRV724-labeled neurons were mostly located in the contralateral oculomotor nucleus (P = 0.0305) (n = 4; Figure 6F–H). The EWN is located at the midline, so there was no comparison between the ipsilateral and contralateral sides (Figure 6I).
Given that the ONC procedure has a considerable impact not only on the survival of RGCs but also on the function and structure of the brain (Zhan et al., 2020; Zhang et al., 2020), we next examined changes in projections from PRV724-labeled RGCs after ONC using optical tissue clearing (Thy1-YFP ONC mice, n = 5). After ONC, we observed fewer PRV724-labeled neurons in the BLA, CeA, and PVH of both hemispheres (Figure 6A). The distribution of PRV724-labeled neurons was decreased after ONC in the ipsilateral BLA (P = 0.0006) and contralateral BLA (control: n = 4 and ONC: n = 5, P = 0.0210, Figure 6B). Furthermore, the distribution of PRV724-labeled neurons was decreased in the contralateral CeA (P < 0.0001), whereas in the ipsilateral CeA (P = 0.0989), we found no significant difference between the control and ONC group (control: n = 4 and ONC: n = 5; Figure 6C). In the PVH of both hemispheres, the distribution of PRV724-labeled neurons was also decreased after ONC (control: n = 4 and ONC: n = 5, ipsilateral P = 0.0440 and contralateral P < 0.0001; Figure 6D). Moreover, the distribution of PRV724-labeled neurons was less apparent in the IGL, LGv, oculomotor nucleus, and EWN of both hemispheres (Figure 6E). The distribution of PRV724-labeled neurons in the contralateral IGL was significantly reduced (P = 0.0496), and that of the ipsilateral IGL was also reduced but this was not significant (P = 0.0791) (control: n = 4 and ONC: n = 5; Figure 6F). In the LGv, the distribution of PRV724-labeled neurons was less apparent in both hemispheres (control: n = 4 and ONC: n = 5, ipsilateral P = 0.0019 and contralateral P = 0.0009; Figure 6G). In the oculomotor nucleus, the distribution of PRV724-labeled neurons was only reduced on the contralateral side (P = 0.0418)—changes on the ipsilateral side were insignificant (P = 0.8907) (control: n = 4 and ONC: n = 5, Figure 6H). In the EWN, which is a thin structure in the midline of the brain, the distribution was also reduced (control: n = 4 and ONC: n = 5, P = 0.0020; Figure 6I).
Figure 7|The brain regions that receive projections from RGCs are unchanged after ONC.
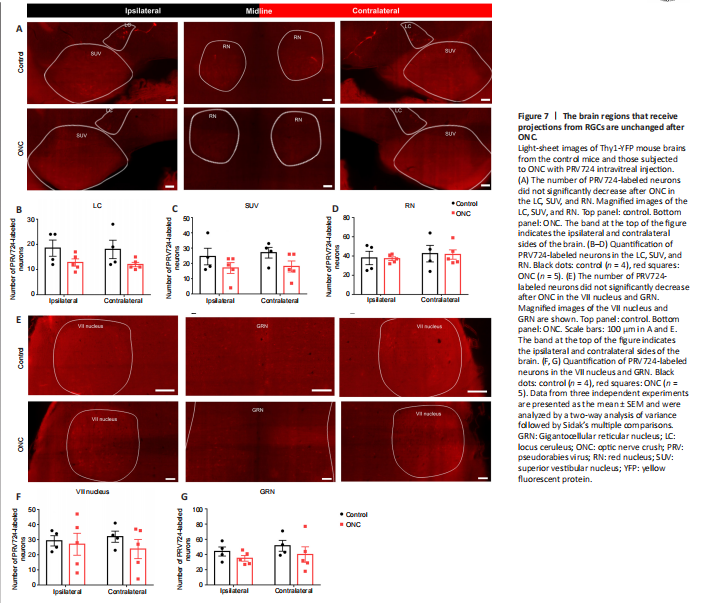
As shown in Figure 7A, the signals of PRV724-labeled neurons were distributed equally across the ipsilateral and contralateral LC (P = 0.9875), SUV (P = 0.8998), and RN (P = 0.8424) (n = 4; Figure 7B–D). As shown in Figure 7E, PRV724-labeled neurons were distributed equally across the ipsilateral and contralateral VII nucleus (P = 0.9418), as well as the GRN (P = 0.7549) (n = 4; Figure 7F and G). Our results confirmed that PRV724-labeled RGCs send more projections to the contralateral PVH, oculomotor nucleus, and CeA.