脑损伤
-
Figure 1|Schematic diagram showing the production of organotypic neural slices, derived from cerebellar tonsillar tissue resected during decompressive surgery for Chiari malformation.
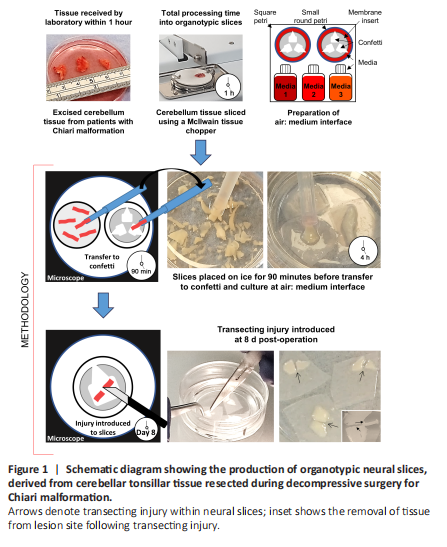
Following published protocols (Weightman et al., 2014), a penetrating, transecting injury was induced in a subset of slices at 8 days post slice derivation with the tissue from within the lesion removed using an aspirator (Figure 1: inset). Lesioned slices were maintained for a further 21 days. Subsets of slices were fixed at 7-, 14- and 21 days post-lesion. Immediately following injury, under microscopic observation the DuraGen PlusTM (~2 mm thickness) was cut to the size and depth of the lesioned tissue and implanted into the lesion site of a subset of slices. DuraGen PlusTM was a kind gift supplied by Integra LifeSciences (Princeton, NJ, USA).
Figure 2|Study outcomes and viability assessment of cerebellar tissue slices.
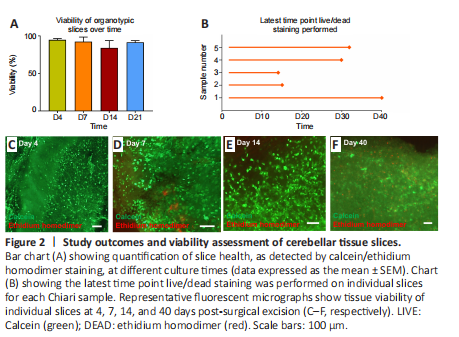
During culture, microscopic observations of tissue provided an indication of overall slice health. Low viability/non-viable slices showed evidence of tissue shrinkage with an opaque, darkened appearance versus the translucent ‘intact’ appearance of healthy tissue slices. Using this method, it was estimated that at 24 hours post-slice derivation, the average number of viable organotypic slices derived from each tissue sample was 45 (approximately 88% of initially derived slices; data not shown). After performing calcein/ethidium homodimer staining, it was observed that tissue viability at each experimental time point was high overall [> ~80% (Figure 2A)]. Slices were observed to be viable for 21 days and in three cases, beyond this time point (Figure 2B–E). In one case, tissue was maintained for 40 days, and viable cells could be observed at this late time point [~72% viable (Figure 2F)].
Figure 3|Major neural cell types contributing to pathology are detectable in cerebellar tissue slices.
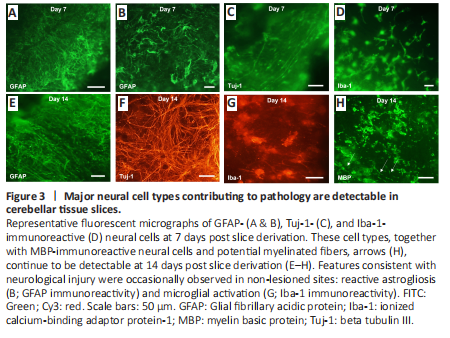
All the major cell types of the CNS could be detected in the slices at 7- and 14-days post tissue derivation (Figure 3). Specifically, neurons (Tuj-1-immunoreactive cells), astrocytes (GFAP-immunoreactive cells), and microglia (Iba-1-immunoreactive cells) (Figure 3A–D) could all be reliably stained and detected, with oligodendrocytes (MBP-immunoreactive cells) also observed on day 14 (Figure 3E–H). Occasionally, cells showing features consistent with reactive astrogliosis [GFAP upregulation and hypertrophy (Figure 3B)] could be observed in non-experimental lesion areas, potentially due to local insult during surgical tissue resection. Morphological changes characterizing microglial activation could be seen at the slice margins on days 7 and 14, with Iba-1-immunoreactive cells showing evidence of rounding with shortened, thicker processes (Figure 3D and G), potentially reflecting immune cell activation during tissue excision from the brain or due to slicing procedures.
Figure 4|A reproducible, penetrating (transecting) lesion can be introduced into the cerebellar tissue slice at 8 days post-slice derivation.、
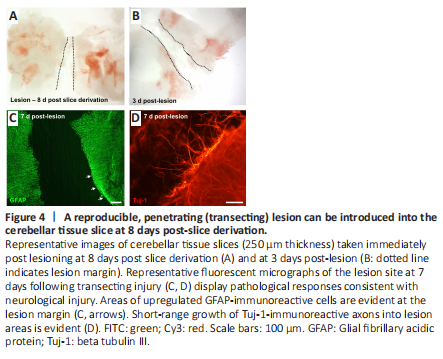
A penetrating (transecting) injury could be reliably introduced into the slices under microscopic guidance, at 8 days post slice derivation (Figure 4A and B). The average lesion width was 342.7 ± 6.7 μm. At 7 days post-lesion, pathological features consistent with reactive gliosis could be observed at the lesion margins as a dense band of GFAP upregulation (Figure 4C). There was also evidence of short-range axonal sprouting, with cellular processes immunostained for Tuj-1 seen extending beyond the lesion edge (Figure 4D).
Figure 5|Integration of cellular tissue and biomaterial occurs at the lesion-DuraGen? Plus interface.
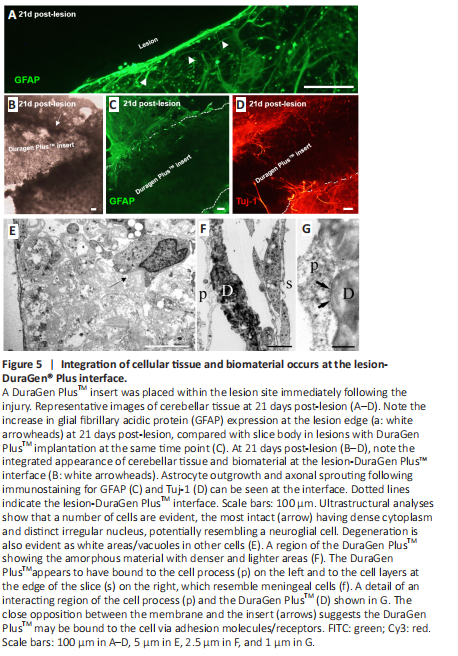
DuraGen PlusTM was implanted into the lesion site immediately following injury. At 21 days post-lesion, in slices without biomaterial implantation, elevated GFAP expression was apparent at the lesion margin (Figure 5A). Cellular integration could be observed at the lesion-DuraGen PlusTM interface (Figure 5B), with infiltration of astrocyte processes immunostained for GFAP (Figure 5C) and short-range sprouting of axons labeled with Tuj-1 (Figure 5D).
Ultrastructural observations of the tissue edge of the lesion adjacent to the DuraGen PlusTM support observations with fluorescence microscopy, that cellular interactions occur at the interface between the slice and biomaterial insert. Individual cell types were difficult to identify at the edge of the lesion due to cellular degeneration. Some intact cells with dense cytoplasm could be observed (Figure 5E), reminiscent of neuroglia, although not definitively so. The DuraGen PlusTM itself was generally amorphous and inhomogeneous, with denser and lighter areas within, but little substructure (Figure 5F). Where the two interacted, cell membranes of adjacent cells were closely opposed to the DuraGen PlusTM, and potentially bound to it by some form of adhesion molecules, for example, cell surface receptors, suggesting the insert had become integrated to a degree with the cells in the slice (Figure 5G). The materials also appeared to interact with cell processes from within the slice and occur underneath the surface, likely meningeal cells in places (Figure 5G).