神经退行性病
-
Figure 1|Induction of chemically-induced neural progenitor cells (ciNPCs) from rat embryonic fibroblasts (REFs) by a chemical cocktail of valproic acid, CHIR99021, and Repsox (VCR) under a physiological hypoxic condition.
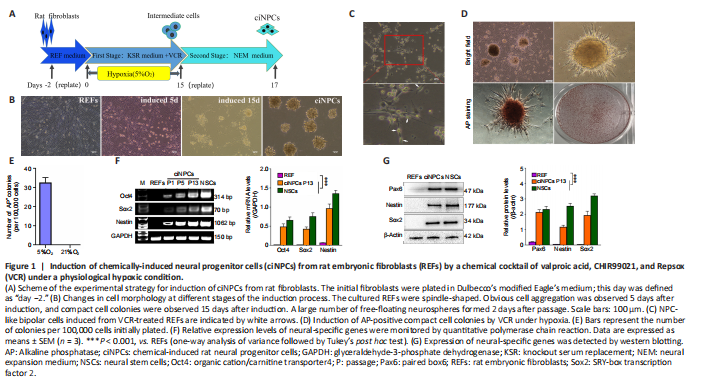
Primary cultured fibroblasts were initially mixed with epithelial cells, and because of their rapid growth, fibroblasts gradually replaced epithelial cells after 2–4 passages of subculture. The induction of ciNPCs from REFs by small molecular compound VCR under physiological hypoxia (5% O2) underwent two major developmental stages (Figure 1A). Step-by-step cell morphological changes throughout the induction process are shown in Figure 1B. During the first stage, the intermediate cells were chemically induced. The cellular morphology of many REFs changed to a bipolar shape, similar to that of NPCs during this process (Figure 1C). Compact cell colonies were observed in REFs cultured at 5% O2 15 days after VCR, and 1000 U/mL leukemia inhibitory factor was added to the culture medium. Approximately 30 compact cell colonies emerged from 1 × 105 REFs, and most colonies (90%) expressed a high level of alkaline phosphatase (Figure 1D and E). The second stage was lineage-specific induction. Intermediary compact cell colonies were digested with 0.25% trypsin-ethylene diamine tetraacetic acid, replated onto low adhesion six-well plates, and cultured in suspension in NEM containing heparin, EGF, and bFGF under normoxic conditions. A large number of free-floating neurospheres were formed 2 days after passage. We collected these free-floating clusters, which we defined as P1 ciNPCs. Additionally, the expression levels of neural-related genes and proteins, including Sox2, Pax6, and Nestin, were significantly enhanced by qPCR and western blot analyses (Figure 1F and G).
Figure 2|Proliferation and self-renewal of chemically-induced neural progenitor cells (ciNPCs).
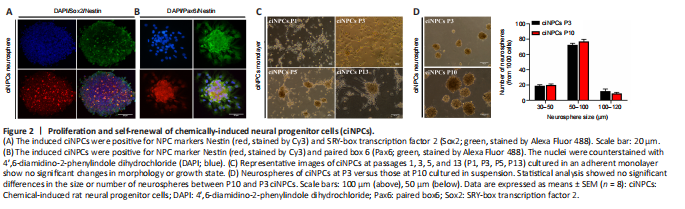
Figure 3|Expression of Nestin, paired box 6 (Pax6), and SRY-box transcription factor 2 (Sox2) was visualized by immunostaining P13 chemically-induced neural progenitor cells (ciNPCs).
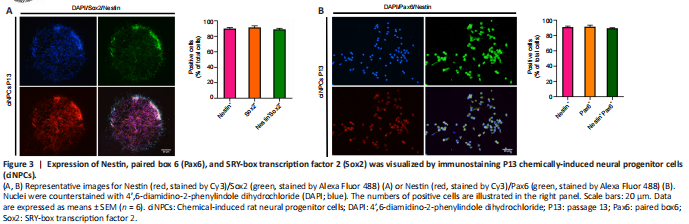
The ciNPC neurospheres induced by VCR under hypoxia were phase-bright and became more spherical as their size increased. The results of immunofluorescence staining showed that the ciNPC neurospheres inoculated on sterile glass slides pre-coated with PLO and laminin had typical neurosphere-like structures that expressed NPC markers, including Nestin, Sox2, and Pax6 (Figure 2A and B). Subsequently, these ciNPC neurospheres were further cultured in suspension to examine whether they possess two fundamental characteristics of NPCs: proliferation and self-renewal. After 13 passages cultured in NEM, the ciNPCs maintained good neurosphere formation and proliferation ability, with no change in morphology. In the different generations (i.e., P1, P3, P5, and P13), the ciNPCs cultured in an adherent monolayer showed no significant changes in morphology or growth state, which was extremely similar to rat NPCs (Figure 2C). Moreover, no significant differences in the size or number of neurospheres were found between P10 and P3 ciNPCs (Figure 2D). Next, we cultured P13 ciNPCs in an adherent monolayer and found that more than 93% of all ciNPCs were stained positive for Nestin, Sox2, and Pax6 individually, and approximately 90% of ciNPCs expressed Nestin/Sox2 or Nestin/Pax6 (Figure 3A and B).
Figure 4|Multipotency of rat embryonic fibroblast (REF)-chemically-induced neural progenitor cells (ciNPCs) in vitro.
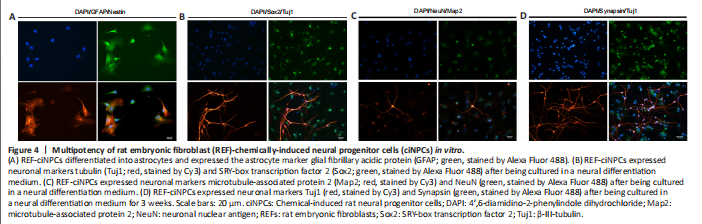
The third fundamental characteristic of NPCs is their developmental multipotency (Cheng et al., 2014). The multipotency of ciNPCs was assessed by culturing cells under neuronal or glial differentiation conditions in vitro. We found that 14 days after induction, most cells of P3 ciNPCs became GFAP-positive with astrocyte-like morphology in neural basal medium with N2 and B27 (Figure 4A). Over 60% of ciNPCs became Tuj1-positive with neuron-like morphology in neural basal medium with B27, N2, BDNF, GDNF, IGF-1, cAMP, and ascorbic acid (Figure 4B). Moreover, neuronal nuclear antigen and postsynaptic microtubule-associated protein 2 double-positive cells with neuron-like morphology were found in the culture (Figure 4C). After being cultured in a neural differentiation medium for 3 weeks, Tuj1-positive mature neurons began to express presynaptic membrane marker synaptophysin (Figure 4D).
Figure 7|Survival and integration of transplanted chemically-induced neural progenitor cells (ciNPCs) in the host brains of the parkinsonian rat models.
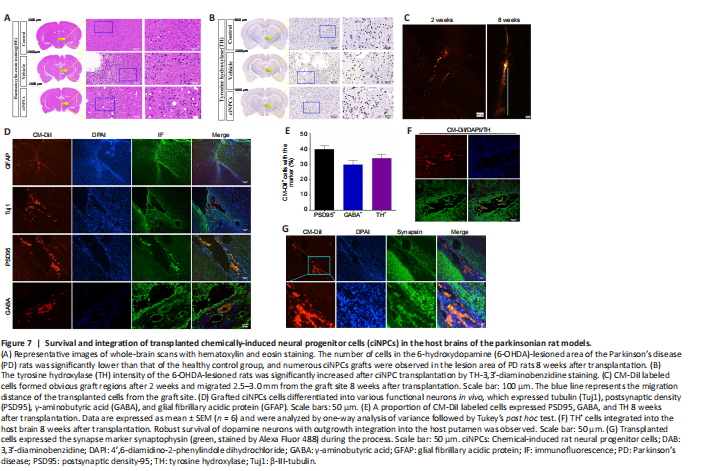
Whole-brain hematoxylin-eosin staining results revealed that the number of cells in the 6-OHDA-lesioned area of PD rats was significantly lower than that of the healthy control group, and the cells were arranged in a disorderly manner. However, numerous ciNPCs grafts were observed and shown to have survived well in the brain environment of PD rats 8 weeks after transplantation (Figure 7A). The TH-3,3′-diaminobenzidine tetrahydrochloride staining of the whole-brain scan revealed significantly fewer TH+ cells in the brain of the vehicle group than in the brain of the healthy control group. However, a large number of TH+ cells were observed in the engraftment area of ciNPCs and the area surrounding the PD rat brain SN 8 weeks after transplantation. Microscopic imaging showed significantly higher TH labeling in the transplanted putamen than in the vehicle group putamen, which indicated that the transplanted putamen recovered well from the transplantation of ciNPCs. Moreover, there were significantly more viable DA neurons in the putamen of the ciNPC group than in the vehicle group (Figure 7B).
Transplanted ciNPCs formed obvious graft regions 2 weeks after implantation and migrated from the graft area 8 weeks after transplantation (Figure 7C). In addition, the area of cell distribution from the grafted area was significantly larger at 8 weeks than at 2 weeks after transplantation. These CM-DiI labeled cells survived for at least 8 weeks and migrated long distances (2.0–2.5 mm) from the graft site into the surrounding brain tissue and damaged area to play a repairing role (Figure 7C). Moreover, the engrafted ciNPCs gave rise to various functional neurons around and within the graft 8 weeks after transplantation and expressed the neuronal marker β-III-tubulin, the glutamatergic neuronal marker PSD95, and the glial marker GFAP (Figure 7D) (Gao et al., 2017). Most CM-DiI labeled cells expressed neuronal markers PSD95, GABA, and TH 8 weeks after transplantation (Figure 7E). These data demonstrate that ciNPCs can differentiate into functional neurons and astrocytes in the host SN. Many grafted cells were positive for TH staining, and TH+ fiber outgrowth was also detected from the graft site. Furthermore, TH+ cell clusters were scattered throughout the graft and integrated into the host brain tissue (Figure 7F). Given that TH is a marker of DA neurons (Parmar et al., 2020), our findings suggest that transplanted ciNPCs can differentiate into DA neurons in the host brain microenvironment. Moreover, the expression of synaptophysin was significantly increased after ciNPC transplantation, and numerous DiI-positive cells were positive for synaptophysin staining (Figure 7G). We also found a large number of synaptophysin-positive and DiI-negative patches adjacent to the transplanted ciNPCs, which indicated that host-derived presynaptic terminals made contact with ciNPCs-derived neurons to form mature synapses (Figure 7G). Importantly, no tumor formation was observed in any of the grafted rats after ciNPC transplantation.