周围神经损伤
-
Figure 3| Effect of let-7a, let-7c, and let-7d on Schwann cell proliferation and migration.
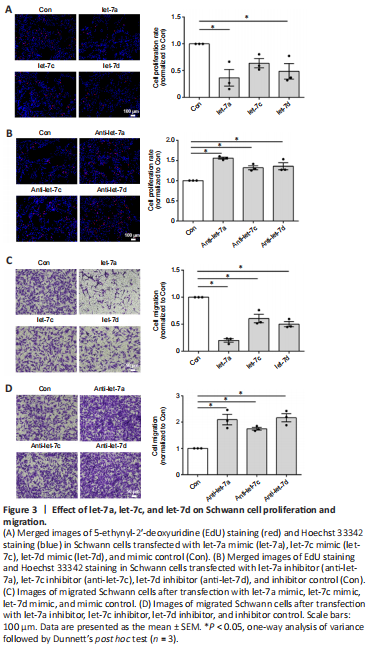
Our previous study demonstrated that let-7d strongly inhibited Schwann cell proliferation and migration (Li et al., 2015b). Here, the functional effects of let-7a and let-7c were also examined. EdU cell proliferation assay showed that Schwann cells transfected with let-7a (P = 0.0110), let-7c (P = 0.0327), or let-7d (P =0.1304) mimic had significantly reduced cell proliferation rates compared with that of the control group (Figure 3A). In contrast, cells transfected with let-7a inhibitor (P = 0.0002), let-7c inhibitor (P = 0.0058), or let-7d inhibitor (P = 0.0030) had elevated cell proliferation rates (Figure 3B). Transwell-based cell migration assay showed that let-7a mimic, let-7c mimic, or let-7d mimic had an inhibitory effect on Schwann cell migration, whereas let-7a inhibitor, let-7c inhibitor, or let-7d inhibitor had a promoting effect on Schwann cell migration (Figure 3C and D). Of these results, let-7a had the most significant effects and anti-let-7a promoted the proliferation and migration of Schwann cells. Taking the results of the endogenous expression and regulatory relationship together, we considered that anti-let-7a was the most suitable molecule for in vivo application from the let-7 family.
Figure 4|Safety assessments of rats injected with let-7a antagomir.
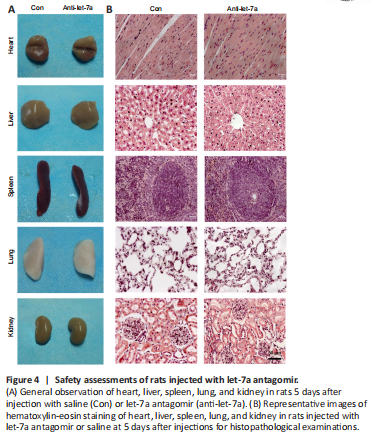
The in vivo application safety of let-7a antagomir was examined by directly introducing a high dose (100 nmol) of let-7a antagomir into rats by caudal vein injection. Morphological characteristics of rat heart, liver, spleen, lung, and kidney were determined by hematoxylin-eosin staining at 5 days after let-7a antagomir injection. The external appearances and weights of these organs in rats injected with let-7a antagomir were similar to those in rats injected with saline only (Figure 4A). Hematoxylin-eosin staining further demonstrated that the histopathological properties of these organs were not affected by let-7a antagomir injection (Figure 4B).
Figure 5|Cellular uptake of the let-7a antagomir.
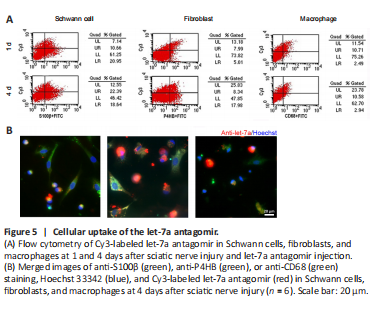
Rat sciatic nerve injury was performed and rat sciatic nerve stumps were subjected to flow cytometry analysis to determine whether let-7a antagomir could enter into cells. At 1 day post-injury, let-7a antagomir-positive ratios in Schwann cells, fibroblasts, and macrophages were approximately 33.7%, 61.5%, and 81.1%, respectively. At 4 days post-injury, let-7a antagomir-positive ratios in Schwann cells, fibroblasts, and macrophages were approximately 54.6%, 31.7%, and 78.3%, respectively (Figure 5A). Immunofluorescence directly showed that let-7a antagomir entered into Schwann cells, fibroblasts, and macrophages (Figure 5B).
Figure 6|Cell compatibility and controlled release of the let-7a antagomir.
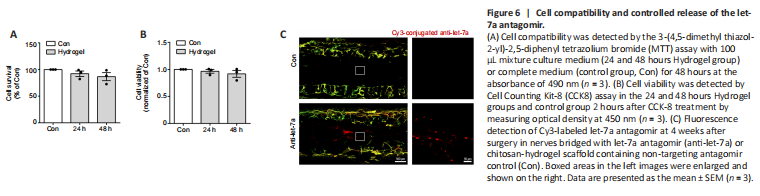
BeaverBanoTM Tissue regeneration and repair hydrogel were used to control release of let-7 antagomir in vivo. Good biocompatibility is essential for hydrogel application in the biomedical field. To investigate cell compatibility of the hydrogel, primary Schwann cells were cultured with complete medium (control group) or hydrogel-soaked culture medium (hydrogel-soaked group). MTT results showed that no significant difference was observed between the hydrogel-soaked culture medium and complete medium (Figure 6A). We further evaluated its effect on the cell viability of Schwann cells. CCK-8 assay showed no significant differences between the Schwann cells cultured in hydrogel-soaked medium and those in the control group (Figure 6B). Hence, the hydrogel is considered as suitable for biomedical applications.
To achieve sustained delivery of antagomir, hydrogel was used as the stabilizer and repository of let-7a antagomir. We evaluated its sustained release effect in vivo. The fluorescent signal of Cy3-labeled was detected at 4 weeks after the nerve grafting, suggesting that sustained release of let-7 antagomir was achieved (Figure 6C).
Figure 7| Immunohistochemistry examinations of rat sciatic nerves.
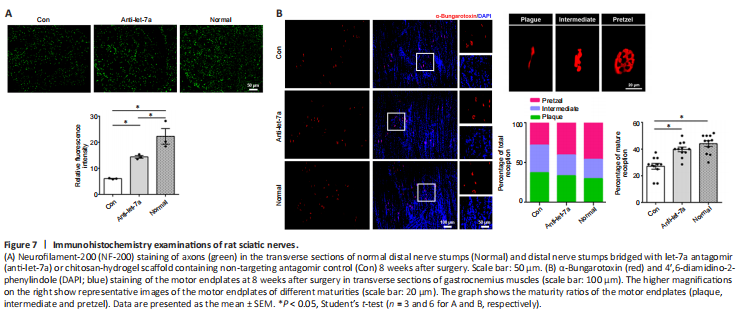
To evaluate its potential application in vivo, we integrated let-7a antagomir into a tissue-engineered nerve graft to bridge the gap of the sciatic nerve injury. The effect of let-7a antagomir on peripheral nerve regeneration was examined by NF-200 staining. Quantitative analysis showed that NF-200 fluorescence in the distal nerve stump in the anti-let-7 group was much lower than that in the normal group, but was significantly higher in the let-7a antagomir group compared to the normal group (P = 0.0264; Figure 7A). The morphology of motor endplates in gastrocnemius muscles observed at 8 weeks showed that in comparison with the normal group, only sparse, immature motor endplates were distributed within the target muscles of the control group (Figure 7B). The percentage of ‘pretzel’ (mature with a weblike pattern) motor endplates was much higher in the let-7a antagomir group than in the control group.
Figure 9|Immunohistochemistry of rat muscles.
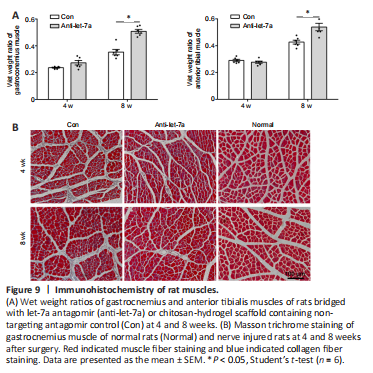
The weight and morphology of the target muscles were also measured. At 4 weeks after surgery, no differences in the wet weight ratios of the anterior tibial muscle and gastrocnemius muscle were found between the control group and the anti-let-7 group. However, at 8 weeks after surgery, the wet weight ratios of the anterior tibial muscle and gastrocnemius muscle in the anti-let-7 group were significantly higher (P < 0.0001) than those in the control group (Figure 9A). Observations from Masson trichrome staining showed that compared with those in the control group, muscle fibers appeared markedly larger and there were fewer collagen fibers in the anti-let-7 group at 4 and 8 weeks after surgery (Figure 9B).