周围神经损伤
-
Figure 2|Protein profiling analysis and the detection of SC function in DPN.
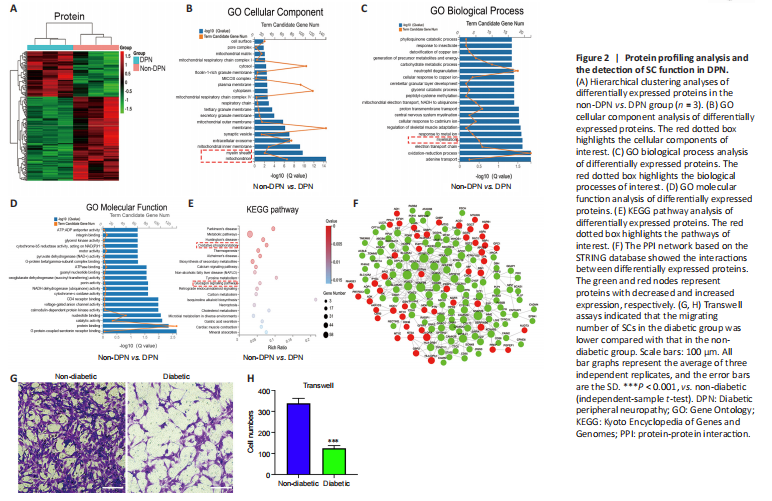
Protein profiling analyses were performed on three pairs of peripheral nerves in the DPN and non-DPN groups. A total of 5353 proteins were identified, and 265 proteins were significantly [P < 0.05, |fold change (FC)| ≥ 1.3] differentially expressed in the DPN group (Additional Table 4), as shown by the hierarchical cluster analysis (Figure 2A). GO cellular component analysis indicated that the differentially expressed proteins were mainly found in the mitochondrion and myelin sheath (Figure 2B and Additional Table 5). GO biological process analysis showed that 390 terms were significantly enriched, among which myelination was potentially related to DPN (Figure 2C and Additional Table 6). The proteins related to myelination were serine incorporator 5, PLLP, gap junction protein gamma 3, proteolipid protein 1, periaxin, and MPZ. GO molecular function analysis showed significant enrichment in G protein-coupled serotonin receptor binding and protein binding (Figure 2D and Additional Table 7). KEGG pathway analysis revealed that 77 pathways were significantly enriched, among which oxidative phosphorylation and the glucagon signaling pathways were potentially related to DPN (Figure 2E and Additional Table 8). Figure 2F shows a protein–protein interaction network constructed according to the differentially expressed proteins and showing the interactions among these proteins. These results indicate that abnormal myelination might play an important role in the pathogenesis of DPN.
Myelin is composed of SCs, which are indispensable for the physiological functions of peripheral nerves (Salzer, 2015). Previously, impaired SC migration was reported to contribute to the abnormal myelination and demyelination of peripheral nerves (Anliker et al., 2013; Yi et al., 2019). Thus, we compared the function of SCs from nerves in the DPN and control groups. The primary SCs isolated from the peripheral nerves exhibited a long spindle shape under an optical microscope (Additional Figure 2A). These were confirmed via positive immunofluorescence staining of S100 calcium binding protein B and glial fibrillary acidic protein (Additional Figure 2B). Cell migration assays showed significantly impaired migration of SCs derived from patients with DPN (Figure 2G and H).
Figure 3|Characterization of circ_0002538 and its function in SCs.
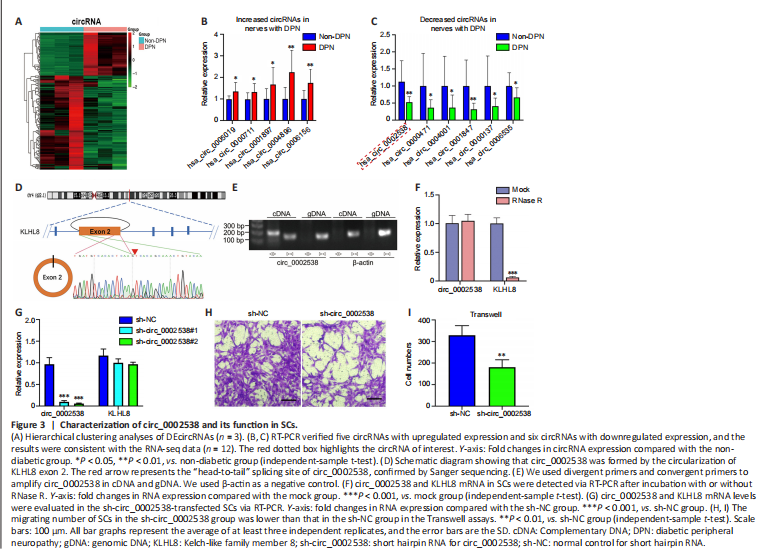
We performed circRNA sequencing for the three pairs of peripheral nerves to uncover their characteristics in the development of DPN. In diabetic peripheral nerves, we identified a total of 15637 circRNAs. A total of 169 circRNAs showed significantly (P < 0.01, q < 0.05, readings ≥ 50, FC ≥ 2) dysregulated expression in the DPN group: 116 circRNAs had significantly downregulated expression and 53 circRNAs had significantly upregulated expression (Additional Table 9). The differentially expressed circRNAs (DEcircRNAs) were directly displayed by hierarchical cluster analysis (Figure 3A). The DEcircRNAs were verified using RT-PCR, and the results showed that six circRNAs with downregulated expression and five with upregulated expression were confirmed in the DPN group (Figure 3B and C). These DEcircRNAs may play an important role in the pathogenesis of DPN.
To further investigate the function of DEcircRNAs in DPN, we focused on circRNA circ_0002538, which showed a 2.14-FC decrease in expression in the DPN group compared with the non-DPN group. circ_0002538 is formed by head-to-tail splicing of exon 2 of the KLHL8 gene, which is located on chromosome 4 (q22.1) (Figure 3D). Sanger sequencing verified the head-to-tail splicing, which was consistent with the data in circBase (Figure 3D). circ_0002538 could be amplified by RT-PCR using divergent primers in cDNA but not in genomic DNA (Figure 3E). circ_0002538 was barely altered after incubation with RNase R comparing to the mock group (Figure 3F), which further confirmed that circ_0002538 has a loop structure.
We confirmed that circ_0002538 expression was decreased in DPN tissues (Figure 3C). Then, we transfected LV-circ_0002538-shRNA into SCs to simulate the pathological process of SCs during DPN. shRNA significantly reduced circ_0002538 expression without affecting the KLHL8 mRNA expression (Figure 3G). We chose sh-circ_0002538 #2 in the following experiments because it had a high inhibitory efficiency compared with the other shRNAs. Migration assays revealed that the knockdown of circ_0002538 impeded the migration of SCs (Figure 3H and I). We further validated the effects of circ_0002538 in the circ_0002538-overexpressing SCs. The expression level of circ_0002538 in these stable overexpression cells was substantially increased, while there was no change in the KLHL8 mRNA level (Additional Figure 3A). Migration assays revealed that the overexpression of circ_0002538 increased the number of SCs that migrated to the lower chamber (Additional Figure 3B and C). These findings indicate that circ_0002538 was involved in regulating SC migration in vitro.
Figure 4|Overexpression of circ_0002538 improves demyelination and symptoms of DPN.
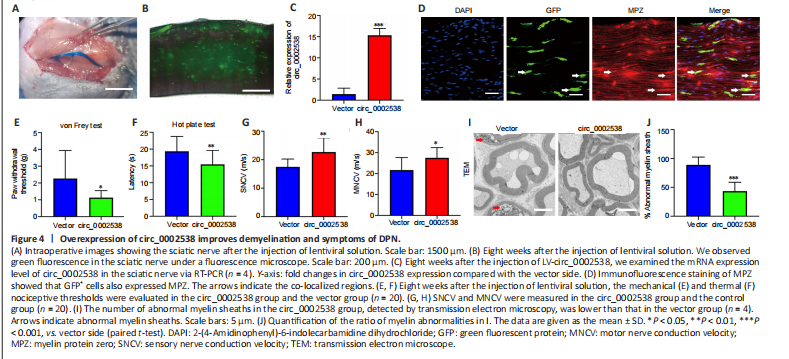
To further assess the role of circ_0002538 in DPN in vivo, we injected circ_0002538 LV into mice with DPN (Figure 4A). We used a fluorescence microscope to examine GFP-positive cells in the sciatic nerve at the 8th week after surgery, and found that injection of the LV-vector led to long-term transgene expression in the sciatic nerve (Figure 4B). RT-PCR revealed that circ_0002538 expression in the circ_0002538 group was higher than that in the vector group (Figure 4C). Immunofluorescence showed that GFP-positive cells also expressed MPZ protein in the circ_0002538 overexpression group, indicating that circ_0002538 was stably expressed in SCs (Figure 4D).
To further examine the effect of circ_0002538 on the signs and symptoms of DPN in vivo, we conducted behavioral tests and neurophysiological measurements. Compared with the control vector group, the circ_0002538 group showed improved thermal and mechanical thresholds (Figure 4E and F). Electrophysiological records showed that compared with those of the control group, the sensory and motor nerve conduction velocities of the circ_0002538 group were significantly increased (Figure 4G and H). These results demonstrated that the upregulation of circ_0002538 expression improved the function of the sciatic nerve in diabetic mice with DPN. Transmission electron microscopy revealed that the percentage of abnormal myelin sheaths, which manifested as myelin infoldings, vacuolization, and uneven thickness, increased in the DPN group but significantly decreased in the circ_0002538 group (Figure 4I and J). These results suggest that the overexpression of circ_0002538 ameliorated the symptoms of DPN by improving myelination.
Figure 6|PLLP regulates SC migration and myelination.
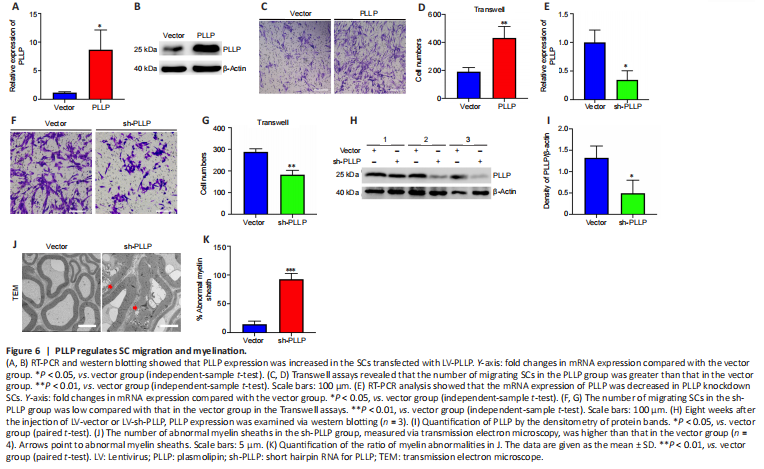
To further verify the role of PLLP in SCs, we transfected a lentiviral vector containing the PLLP gene into SCs. RT-PCR showed that the PLLP overexpression cells had significantly increased PLLP expression, which was further confirmed by the western blotting results (Figure 6A and B). We performed a mRNA-sequencing analysis of the SCs transduced with the LV carrying either PLLP or the control vector. A total of 23448 mRNAs were identified, and 1671 mRNAs met the filtering criteria (P < 0.05, FC ≥ 2) (Additional Table 10). The filtered mRNAs were further analyzed using GO analysis for functional prediction (Additional Tables 11–13). GO biological process analysis showed that the filtered mRNAs were significantly enriched in neutrophil migration, regulation of neutrophil migration, positive regulation of neutrophil migration, and positive regulation of leukocyte migration, indicating that PLLP might be related to cell migration (Additional Figure 4A). Transwell assays confirmed that the overexpression of PLLP significantly increased SC migration (Figure 6C and D). We further validated the role of PLLP by knocking it down. RT-PCR revealed that PLLP expression was decreased in PLLP knockdown SCs (Figure 6E). Transwell assays showed that the knockdown of PLLP effectively inhibited SC migration (Figure 6F and G). These results indicate that PLLP affects SC migration.
To verify the effect of PLLP on peripheral nerve myelination in vivo, we injected sh-PLLP LV into the mouse sciatic nerve. Western blotting revealed that PLLP was decreased in the PLLP knockdown group compared with the control vector group (Figure 6H and I). The ratio of myelin abnormalities was strongly increased in the PLLP knockdown group, as shown by transmission electron microscopy (Figure 6J and K). These results indicate that PLLP might regulate myelination in peripheral nerves.
Figure 8|miR-138-5p inhibits SC migration by targeting PLLP.
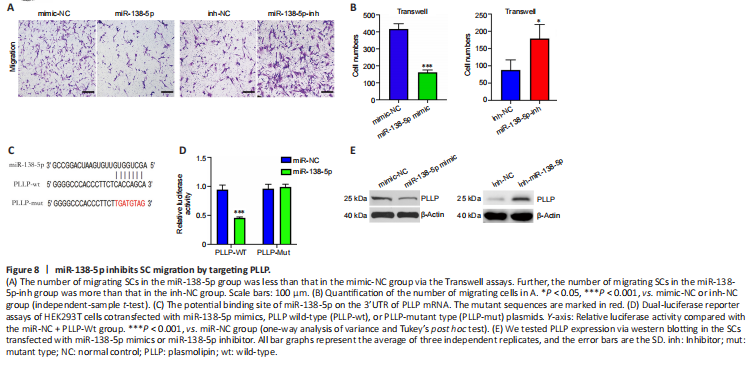
To investigate the function of miR-138-5p, we transfected miR-138-5p mimic or inhibitor into SCs. In the migration assays, the number of SCs that migrated to the lower chamber was significantly reduced after transfection with the miR-138-5p mimics. In contrast, the miR-138-5p inhibitor enhanced SC migration (Figure 8A and B). Then, we used a dual-luciferase reporter assay to determine whether miR-138-5p could bind to PLLP to regulate its expression. Figure 8C shows the predicted binding sites and mutated sites of miR-138-5p on the 3′UTR of PLLP. The overexpression of miR-138-5p significantly weakened the relative Rluc activity of the wild-type plasmids but not the mutant plasmids (Figure 8D), suggesting that miR-138-5p could directly bind to the PLLP 3′UTR and block its activity. Western blot analysis further demonstrated that the miR-138-5p mimics significantly reduced PLLP protein expression, while the miR-138-5p inhibitors increased PLLP protein expression (Figure 8E). These results revealed that miR-138-5p could strongly suppress SC migration by targeting PLLP.
Figure 9|miR-138-5p reverses the circ_0002538-mediated promotion of SCs.

We demonstrated that circ_0002538 could sponge miR-138-5p and that miR-138-5p could inhibit SC migration by targeting PLLP. Subsequently, we explored whether circ_0002538 could regulate PLLP through miR-138-5p. The SCs cotransfected with the miR-138-5p mimics and circ_0002538 exhibited decreased migration compared with the SCs transfected with circ_0002538 only (Figure 9A and B), which indicated that ectopic expression of miR-138-5p could partially eliminate the promoting effect of circ_0002538. Western blot analysis showed that the SCs cotransfected with the miR-138-5p mimic and circ_0002538 exhibited reduced PLLP expression compared with the SCs transfected with circ_0002538 only (Figure 9C and D). The above results demonstrated that circ_0002538 regulated SC migration in part by sponging miR-138-5p and subsequently influencing PLLP expression.