周围神经损伤
-
Figure 1|PTEN expression increases in cochlear cells after noise exposure.
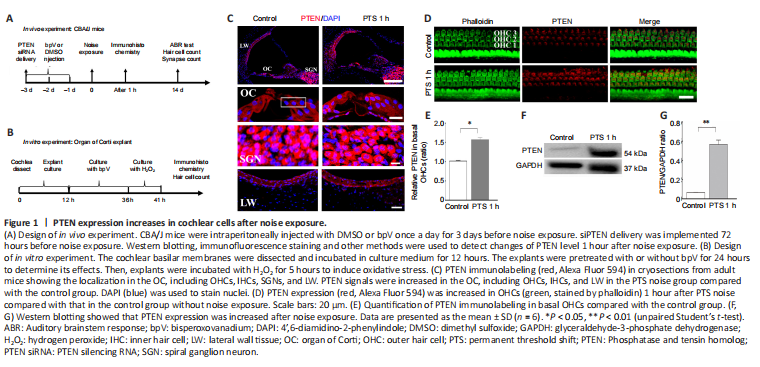
First, we detected PTEN expression levels in cryosections by immunostaining 1 hour after noise exposure. PTEN signals were increased in the organ of Corti, including OHCs and IHCs, and in the nuclei of marginal cells in lateral wall tissues, but did not change in the nuclei of spiral ganglion neurons (Figure 1C). Noise exposure led to an elevation of PTEN level in OHCs and a decreased number of OHCs, which indicated that the model of permanent hearing impairment was successfully established (Figure 1D). Quantitative analysis confirmed that PTEN levels increased significantly in basal OHCs exposed to noise compared with the control cells (P = 0.011; Figure 1E). We then evaluated PTEN expression in entire cochlear homogenate by western blotting and found that PTEN expression was elevated 1 hour after noise exposure (Figure 1F). Quantification showed PTEN expression level after noise exposure was more than 6-fold the control level (Figure 1G).
Figure 2|PTEN inhibitor bpV attenuates noise-induced changes in hearing threshold, loss of hair cells and synaptic ribbons.
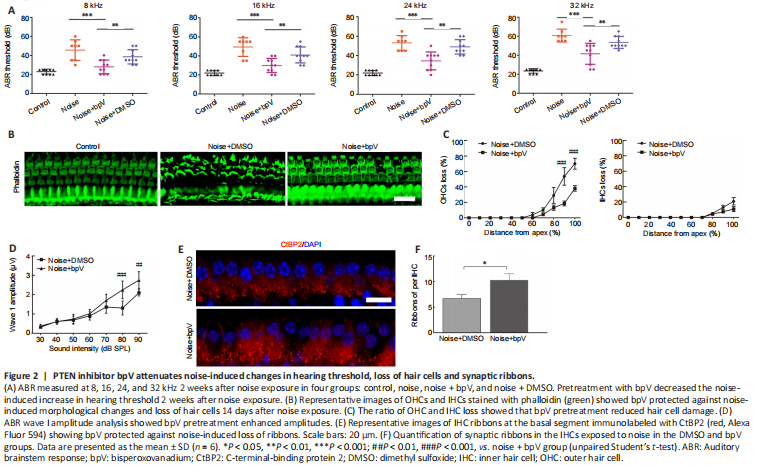
To investigate the role of PTEN in NIHL, we first evaluated the impact of PTEN inhibitor bpV on noise-induced hearing threshold. We subjected CBA/J mice to 105 dB noise exposure for 2 hours, which significantly triggered acoustic trauma and hair cell loss. ABR measurements showed that bpV pretreatment attenuated the auditory threshold at 8, 16, 24, and 32 kHz, whereas DMSO had little effect on hearing impairment following 14 days of noise exposure (Figure 2A). Morphological analysis indicated severe injury of OHCs in the DMSO group, whereas fewer OHCs were lost in the bpV-pretreated group 14 days after noise exposure (Figure 2B). Noise trauma mainly damaged basal OHCs, which corresponds to hearing loss at higher frequencies. OHC and IHC loss began at a 50% and 65% distance from the apex, respectively. At the end of the basal region, OHC and IHC loss was 75% and 20%, respectively. Further analysis showed the ratio of OHC and IHC loss was reduced in the bpV-pretreated group compared with the DMSO group, suggesting that bpV suppressed noise-induced hair cell loss, especially for OHCs (Figure 2C).
We also measured the ABR peak I amplitudes at click and found that the bpV treatment group had higher wave I amplitudes for sound stimuli ranging from 50 to 90 dB sound pressure level compared with those of the DMSO group 14 days after the noise exposure (Figure 2D). Considering that the ABR wave I amplitude represents auditory nerve fiber activity, differences in wave I amplitudes indicate changes in synaptic function (Liu et al., 2019). We next counted the number of presynaptic ribbons immunolabeled with CtBP2. In accordance with the ABR wave I amplitude analysis, we found that bpV pretreatment reduced noise-induced loss of IHC synaptic ribbons in the apical turn region 14 days after noise exposure compared with DMSO treatment (Figure 2E and F).
Figure 3|bpV prevents noise-induced reductions in PI3K-Akt signaling in OHCs.
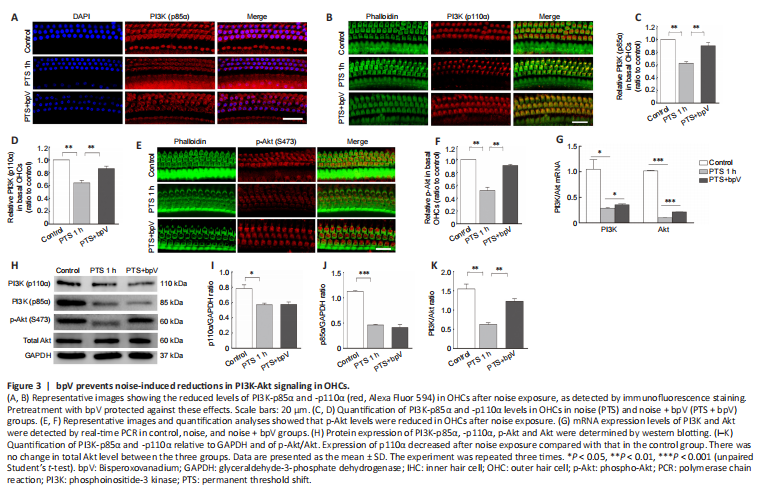
Because PTEN regulation of PI3K-Akt signaling is related to the endogenous protection of cochlear hair cells, we hypothesized that the mechanism of bpV protection against NIHL occurs via the PI3K-Akt signaling pathway. We observed that noise exposure resulted in reductions in PI3K-p85α and PI3K-p110α levels in OHCs, and pretreatment with bpV reversed this effect (Figure 3A and B). Quantitative analysis showed that p85α (P = 0.007) and p110α (P = 0.009) levels in OHCs were higher in the bpV-pretreated group than in the PTS noise exposure group (Figure 3C and D).
Phosphorylation level of Akt was decreased in OHCs after noise exposure, and p-Akt levels were recovered by pretreatment with bpV (Figure 3E and F). We also examined the mRNA levels and found that the bpV-treated group had increased expression of PI3K-Akt survival signal compared with that of the noise exposure group (Figure 3G). In addition, western blotting showed that p85α, p110α and p-Akt expression were decreased in cochlear homogenate after noise exposure compared with controls (Figure 3H). Treatment with bpV increased p85α and p110α levels in OHCs compared with those in the PTS group, but had no significant effect on p85α and p110α expression levels (Figure 3I and J), suggesting that p85α and p110α were also localized in other types of cochlear cells such as Deiters cells. Furthermore, bpV pretreatment increased the noise-induced ratio of p-Akt/total-Akt (Figure 3K).
Figure 4|Intratympanic delivery of PTEN siRNA attenuates noise-induced PTEN expression and hearing threshold changes and upregulates p85α level.
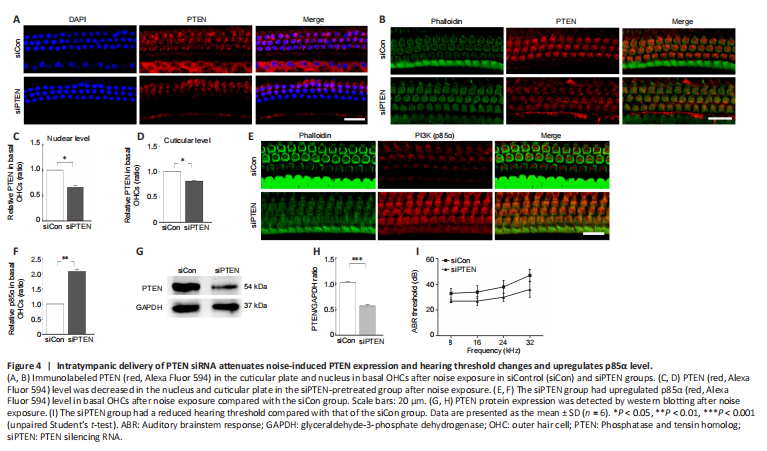
PTEN inhibitor bpV ameliorated NIHL pathology and upregulated the PI3K-Akt signal. To further ascertain the function of PTEN in NIHL, intratympanic delivery of siPTEN was conducted, which showed effects on cochlear hair cells. Immunolabeling for PTEN in basal OHCs both at the cuticular plate and the nucleus were evaluated following siPTEN pretreatment (Figure 4A and B). The relative expression of PTEN was decreased in both the cuticular plate and the nucleus in the siPTEN-pretreated group compared with the siControl group (Figure 4C and D). To further demonstrate that PTEN reduction activated the PI3K-Akt survival signal, we also investigated the p85α level in OHCs and found that p85α level was increased in the siPTEN group compared with that in the siControl group (Figure 4E and F). The silencing efficiency was assessed by western blotting; analysis of the PTEN band indicated a 50% reduction after treatment with siPTEN (Figure 4G and H). Furthermore, the siPTEN group had a lower auditory threshold, especially at 32 kHz (a difference of approximately 10 dB), after noise exposure compared with that of the siControl group (Figure 4I).
Figure 5|bpV prevents H2O2-induced hair cell loss by reducing oxidative stress in cochlear explants.
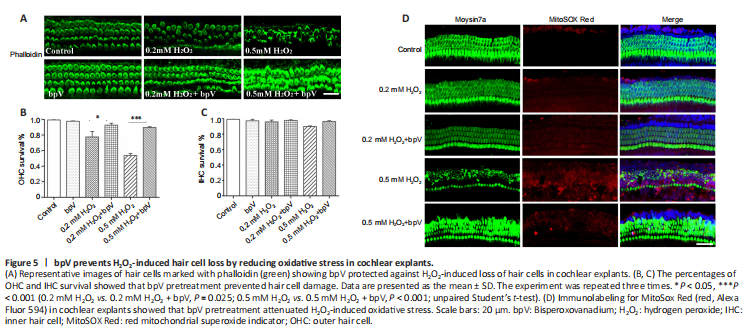
Oxidative stress has been well documented as a main pathophysiological mechanism of NIHL and results in cochlear hair cell dysfunction and eventual death (Xiong et al., 2021). To explore whether the protective effect of bpV against noise-induced hair cell damage involved a reduction in oxidative stress, we used H2O2 as an oxidizing agent to induce oxidative damage in cochlear explants. Hair cells in the control and bpV groups were well preserved, suggesting that bpV has no toxicity. The explants showed OHC loss when treated with H2O2, and treatment with bpV restored a normal appearance of the H2O2-treated OHCs (Figure 5A). Hair cell morphology along the entire explants was observed, and H2O2 mainly induced damage in the basal region of OHCs, with no apparent change in IHCs. Quantitative assessment of hair cells showed 50% and 80% OHC survival with 0.5 mM and 0.2 mM H2O2 treatment, respectively, and bpV increased the survival of hair cells (Figure 5B and C).
Next, we evaluated mitochondrial ROS generation in cochlear explants by MitoSOX Red to determine whether bpV reduced H2O2-induced oxidative stress. We observed a low level of MitoSox Red fluorescence in hair cells treated with 0.2 mM H2O2, and the level was unchanged by pretreatment with bpV. Stronger fluorescence was observed with the higher H2O2 concentration of 0.5 mM, which suggests that a high ROS level leads to serious hair cell injury. In addition, MitoSOX Red fluorescence intensity was significantly reduced by bpV pretreatment when compared with that of the 0.5 mM H2O2 group (Figure 5D). To confirm that PTEN inhibitor bpV protects against NIHL by reducing oxidative stress, we further assessed the expression of 4HNE, a marker of oxidative stress (He et al., 2021), by performing immunolabeling on cochlear surface preparations. Notably, noise exposure increased 4HNE levels in OHCs, and bpV pretreatment decreased 4HNE expression (Additional Figure 1).