脑损伤
-
Figure 1|NOX4 expression reaches the peak at 72 hours in the brain after intracerebral hemorrhage (ICH) in rats.

After establishment of the rat ICH model, the hemorrhagic focus was evaluated at different time points of ICH by MRI. The strongest edema-dominant effect on the tissue around the hemorrhagic foci was present around 72 hours after ICH; the edema and occupying effect were reduced after 72 hours (Figure 1A). To identify whether NOX4 expression after ICH is related to the edema occupying effect around the bleeding foci, we evaluated NOX4 expression at different time points in the brain of rats in the same group by immunofluorescence staining. NOX4 expression was the highest around 72 hours and was proportional to the magnitude of the edema occupying effect around the hemorrhagic foci (Figure 1A). To confirm the timing of NOX4 expression increase, western blotting and immunohistochemical staining were performed. The results showed that NOX4 expression was the highest at 72 hours after ICH in rats (Figure 1B–E).
Figure 2|NOX4 knockdown relieves oxidative stress and enhances the neuronal tolerance to oxidative stress after intracerebral hemorrhage (ICH).

Our results showed that NOX4 expression increased after ICH. To investigate the role of NOX4 function after ICH, we used AAV expressing shRNA against NOX4 to knockdown the expression of NOX4 (Additional Figure 2A–G). We then performed ICH modeling in rats injected with AAV-NOX4 (1 × 1013 IU/μL, 2 μL, 2 weeks) (Figure 2A and B) and confirmed the reduction in NOX4 mRNA levels in the AAV-NOX4 group (Figure 2C).
Increased NOX4, which is the main source of ROS, causes oxidative stress reaction in the brain (Jung et al., 2016). Compared with the sham group, the ICH group showed markedly increased ROS level in the brain, while AAV-NOX4 prevented the up-regulation of ROS (Figure 2D). Our results showed that NOX4 was most significantly elevated in neurons after ICH. Therefore, we next examined if NOX4 knockdown improved the neuronal tolerance to oxidative stress after ICH by performing western blot analysis for antioxidant proteins Nrf2 and Keap-1. Nrf2 and Keap-1 levels were both decreased in rat brain after ICH, and their expressions were increased with NOX4 knockdown (Figure 2E–G). Immunofluorescence staining revealed that the immunopositivity of Nrf2 in neurons of ICH rat brain treated with AAV-NOX4 was increased compared with that in the ICH + AAV-CON group (Figure 2H and I). Therefore, these findings indicated that NOX4 knockdown inhibits ROS production and oxidative stress and improves the neuronal tolerance to oxidative stress after ICH.
Figure 3|Knockdown of NOX4 reduces cerebral edema and neuronal pyroptosis after intracerebral hemorrhage (ICH).

We next examined the effects of NOX4 knockdown on neuronal and neurological function injury following SBI after ICH. MRI of rats treated with AAV-CON or AAV-NOX4 was performed (Figure 3A) and the brain water content of rats were measured (Figure 3B). The results showed that the edematous area around the bleeding lesion and the water content of the rat brains with ICH were reduced after NOX4 knockdown. The occurrence and development of cerebral edema is closely related to the permeability of the BBB (Zhang et al., 2020). Therefore, we used Evans blue staining to test BBB permeability at 72 hours after ICH. The leakage of Evans blue dye in the NOX4 knockdown group was significantly reduced, indicating that NOX4 knockdown alleviated the damage to the BBB permeability after ICH (Figure 3C and D).
Considering that NOX4 was mainly increased in the neurons after ICH, we performed Nissl staining. The results showed that ICH disrupted the normal form of neurons, while NOX4 knockdown prevented these effects and downregulated the number of Nissl bodies (Figure 3E and F). We further assessed neurological function after NOX4 knockdown in rats using mNSS. We found that NOX4 knockdown effectively prevented neurological deficit caused by ICH in rats (Figure 3G).
A previous study demonstrated a role for NOX4 in reducing neuronal apoptosis (Kleinschnitz et al., 2010), but whether NOX4 is involved in other types of cell death is unknown. Unlike apoptosis (Luo et al., 2022), during pyroptosis, the nucleus does not shrink and the cells swell and blister until their membranes rupture (Li et al., 2020). We extracted brain tissues before and after ICH for TEM analysis and found that the neurons in ICH model rats showed obvious signs of pyroptosis, however, this injury was not observed in the AAV-NOX4 group (Figure 3H). There was no significant change in the nucleus of neurons after ICH, but the cell membrane ruptured and the cytoplasmic contents poured out; in contrast, the cell membranes of rats treated with AAV-NOX4 were intact. We further extracted and cultured primary neurons before and after treatment. Under the light microscope, the cell membranes of the neurons in the ICH group exhibited a bubbling phenomenon, while most cell membranes of the neurons in the AAV-NOX4 group were smooth and intact (Figure 3I). Together, these findings demonstrated that knockdown of NOX4 alleviated SBI after ICH, including cerebral edema, neurological impairment and neuronal pyroptosis.
Figure 4|Knockdown of NOX4 ameliorates neuronal pyroptosis through caspase 1/GSDMD-N and caspase4/11/GSDMD-C pathways after ICH.

We demonstrated that NOX4 knockdown can reduce neuronal pyroptosis after ICH, but the exact pathways were unknown. We examined the expression of pyroptosis-related proteins by western blot and found that the pyroptosis promoter protein caspase1, specific protein caspase4/11 and GSDMD were significantly increased after ICH (Figure 4A–H), especially caspase4/11, which was cleaved after ICH and the cleaved protein expression was significantly increased. Levels of the N-terminal and C-terminal of the pyroptosis-specific protein GSDMD were significantly increased after ICH. All these phenomena were reversed with NOX4 knockdown. We also detected the expression of GSDMD in neurons by immunofluorescence staining and immunohistochemical staining (Figure 4I–L). GSDMD expression in neurons was elevated after ICH, whereas the expression of GSDMD tended to be normal with NOX4 knockdown.
Figure 5|NOX4 knockdown reduces the NOX4 expression and reactive oxygen species (ROS) production in mitochondria, and improves neuronal mitochondrial function after intracerebral hemorrhage (ICH).
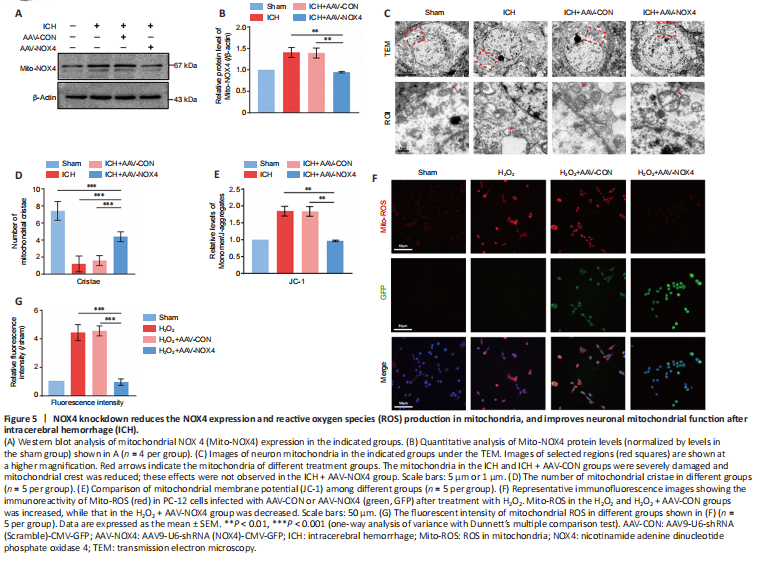
Mitochondria are the main source of intracellular ROS and produce approximately 90% of ROS in cells (Zhang et al., 2019; Zhang and Wong, 2021). A previous study only measured NOX4 and ROS levels in tissues (Morioka et al., 2018). To further accurately assess the changes of NOX4 and ROS in mitochondria after ICH, we extracted mitochondria from tissues and performed western blot. The results showed that NOX4 expression in mitochondria (Mito-NOX4) was increased after ICH, and AAV-NOX4 successfully reduced NOX4 expression in the mitochondria, consistent with the overall NOX4 expression trend (Figure 5A and B). TEM was used to observe the changes in the mitochondria of neurons of different treatment groups. The mitochondria were severely damaged after ICH, with loss of mitochondrial shape, and the number of mitochondrial cristae was severely reduced; these effects were not observed in the AAV-NOX4 treatment group (Figure 5C and D). We further investigated the changes in the mitochondrial membrane potential using JC-1. The results showed that the ratio of JC-1 monomer/polymer in the mitochondria increased after ICH, indicating mitochondrial membrane depolarization and mitochondria damage; in the AAV-NOX4 group, these effects were not observed (Figure 5E).
Because mitochondrial ROS content in brain tissues could not be specifically detected, we simulated a cell model after oxidative stress stimulation. PC-12 cells, commonly used to simulate neuron cell lines (Liu et al., 2020), were transfected with AAV-NOX4 and stimulated by H2O2, followed by staining with MitoTracker Red CMXRos. The fluorescence intensity of MitoROS in PC-12 cells transfected with AAV-NOX4 was significantly lower than that in the oxidative stress treatment group, which was consistent with the previous results (Figure 5F and G). Together, these results suggested that NOX4 knockdown reduced NOX4 in the mitochondria and reduced ROS expression, thus improving neuronal mitochondrial function after ICH.
Figure 6|Mito-TEMPO reduces neuronal pyroptosis and oxidative stress response after intracerebral hemorrhage (ICH).

Evans blue staining revealed that Mito-TEMPO had the same improvement effect on BBB permeability as observed in the AAV-NOX4 group after ICH (Figure 6A and B). To determine whether the inhibitory effect of Mito-TEMPO on ROS is related to NOX4 in mitochondria, western blot analysis was performed, the results showed that Mito-TEMPO reduced NOX4 expression in the mitochondria (Figure 6C and D). Nissl staining showed that the death of neurons was reduced by Mito-TEMPO after ICH, similar to the AAV-NOX4 group (Figure 6E and F). Numerous studies have reported that mitochondrial H2O2 production is induced by NOX4 (Hirschh?user et al., 2015). We also evaluated mitochondrial H2O2 content in the brain tissues after ICH and found that the H2O2 content in the mitochondria of brain tissues was reduced in both AAV-NOX4 and Mito-TEMPO groups after ICH (Figure 6G). Finally, we compared the improvement in neuronal pyroptosis after AAV-NOX4 and Mito-TEMPO treatment and observed that both treatment groups demonstrated reduced expression of GSDMD after ICH, indicating that the effect of AAV-NOX4 and Mito-TEMPO on neuronal pyroptosis was equivalent (Figure 6H and I). Therefore, Mito-TEMPO can reduce neuronal pyroptosis and mitochondria oxidative stress after ICH, and NOX4 may be an important potential target of Mito-TEMPO.
点击此处查看全文