神经退行性病
-
Figure 3|Effects of exendin-4 and linagliptin on dopaminergic neurons in the SN and cell terminals in the STR.
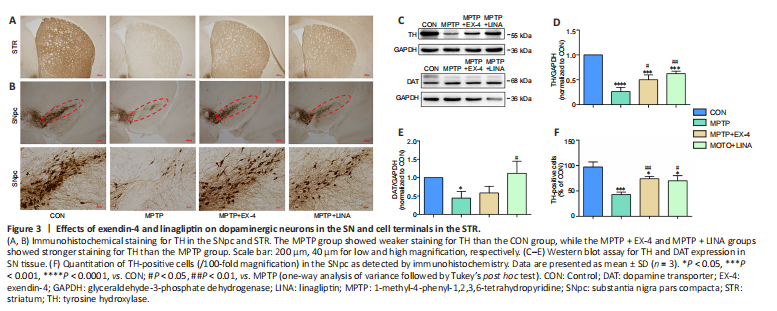
One of the hallmark pathological changes in patients with PD is the progressive death of dopaminergic neurons. TH is the key enzyme involved in dopamine synthesis and can be used as a biomarker for dopaminergic neurons . Immunohistochemical staining for TH-positive cell bodies in the SN pars compacta and cell terminals in the striatum showed a severe loss of dopaminergic neurons caused by MPTP. However, treatment with exendin-4 or linagliptin protected against dopaminergic neuron loss (Figure 3A and B). Furthermore, the western blot results demonstrated that TH and DAT expression levels were decreased in the SN tissue of mice in the MPTP group, whereas a significantly restoration of TH and DAT expression was observed in the exendin-4 and linagliptin treatment groups (Figure 3C–E). Finally, immunohistochemistry-based quantification of TH-positive cells in the SN pars compacta demonstrated that MPTP-induced dopaminergic neuron death in the SN pars compacta was attenuated in the exendin-4 and linagliptin treatment groups (Figure 3F).
Figure 4|Effects of exendin-4 and linagliptin on activation of microglia and astrocytes in the SN.

Chronic neuroinflammation plays a key role in dopaminergic neuron death (Calabrese et al., 2018). To study the effects of exendin-4 and linagliptin on neuroinflammation, we conducted immunohistochemical staining for Iba1 and GFAP, which label microglia and astrocytes, respectively. The results showed that in the SN, especially in the SN reticular region, the number of microglia and astrocytes in the MPTP group was significantly increased, and the cell morphology was consistent with activation (Krashia et al., 2019). Activated microglia appear amoeba-like, with enlarged cell bodies and unclear branches. Activated microglia can further activate astrocytes, resulting in neurotoxicity (Liddelow et al., 2017; Figure 4A and B). However, the number of microglia and astrocytes was markedly decreased in both the exendin-4 and the linagliptin treatment groups (Figure 4C and D).
Figure 5|Effects of exendin-4 and linagliptin on microglial polarization in the SN.
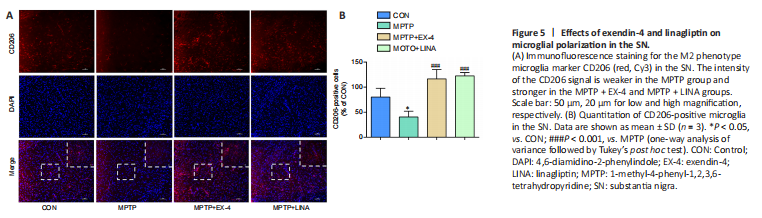
Under normal conditions, microglia remain in a resting state. When exposed to different external stimuli, microglia can polarize into the M1 or M2 phenotype (Pisanu et al., 2014; Joers et al., 2017). Immunofluorescence staining was performed for CD206, a surface biomarker of M2 microglia. The results showed that the number of anti-inflammatory M2 microglia was clearly reduced in the SN of mice in the MPTP group, whereas the number of anti-inflammatory M2 microglia was increased in the exendin-4 and linagliptin treatment groups (Figure 5A and B).
Figure 9|Effects of exendin-4 and linagliptin on the viability of SH-SY5Y cells treated with MPP (+).
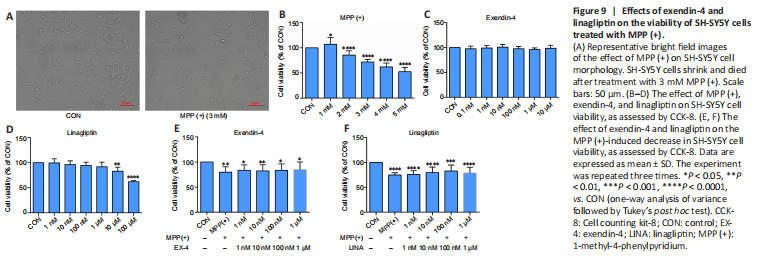
We used SH-SY5Y cells to simulate dopaminergic cells to establish PD models in vitro. The CCK-8 assay results showed that SH-SY5Y cell viability decreased to 70% when treated with 3 mM MPP (+) (Figure 9A and B), and thus this concentration was used in all subsequent experiments. Then, we determined the safe concentration range of exendin-4 and linagliptin for the rescue experiments (Figure 9C and D). Half an hour before adding 3 mM MPP (+) to the cells for the CCK-8 experiment, the drugs were added at different concentrations, and the cells were incubated for 24 hours. The results showed that neither of the two drugs directly rescued the decline in SH-SY5Y cell viability caused by MPP (+) (Figure 9E and F). To further explore the effects of exendin-4 and linagliptin on SH-SY5Y cells, we treated cells with the optimized concentrations of exendin-4 and linagliptin and assessed cell apoptosis by flow cytometry. The results showed that the two drugs had no effect on MPP (+)-induced late apoptosis, which was consistent with the results from the CCK-8 experiment. However, there was a significant increase in the number of early apoptotic cells in the MPP (+) group, and only 100 nM linagliptin slightly reduced the number of early apoptotic cells induced by MPP (+) (Figure 10).
Figure 11|Effects of exendin-4 and linagliptin on MPP (+)-induced NLRP3 expression in BV2 cells.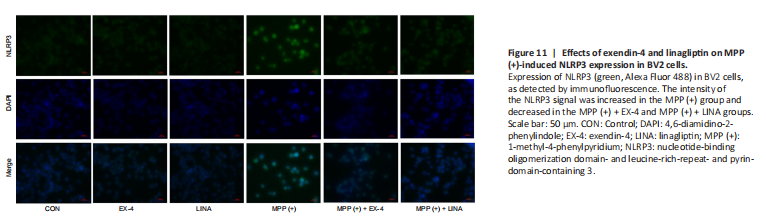
Figure 12|Effects of exendin-4 and linagliptin on MPP (+)-induced ROS production in BV2 cells.
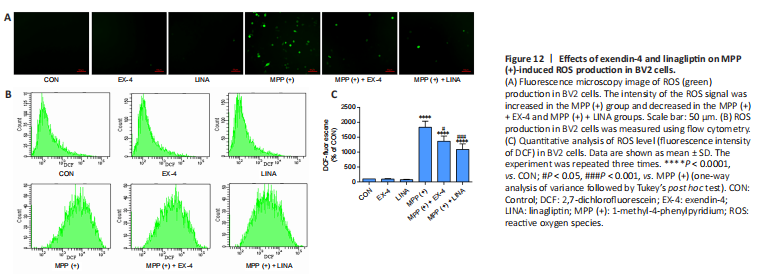
To further investigate the anti-inflammatory properties of exendin-4 and linagliptin in vitro, we detected NLRP3 expression in BV2 cells. MPP (+) treatment significantly increased NLRP3 expression in BV2 cells, while exendin-4 and linagliptin inhibited NLRP3 expression (Figure 11). Then, we used flow cytometry to analyze intracellular ROS levels in BV2 cells. MPP (+) is the toxic metabolite of MPTP, and MPP (+) treatment significantly increases ROS accumulation in BV2 cells (Schildknecht et al., 2017). However, co-treatment with exendin-4 or linagliptin significantly reduced the ROS levels (Figure 12 and Additional Figure 2). Furthermore, intracellular ROS levels were lower in the linagliptin treatment group than in the exendin-4 treatment group.