神经损伤与修复
-
Figure 1|Optimized method for the rapid rescue and preparation of the CVS-N2c-ΔG virus.
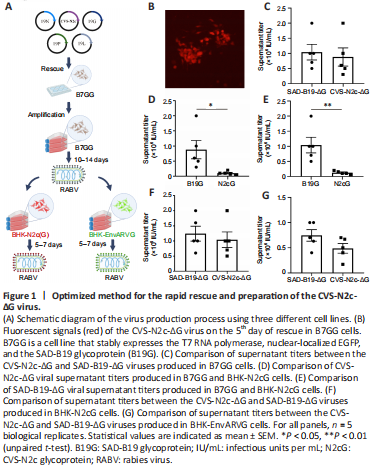
CVS-N2c-ΔG exhibits significantly enhanced retrograde trans-synaptic ability and reduced toxicity to neurons compared with the SAD-B19-ΔG vaccine strain, and it can express functional probes such as calcium-sensitive probes and optogenetic probes for analysis of the functional network (Reardon et al., 2016). However, it has disadvantages of a prolonged preparation process and a low yield. Therefore, it is necessary to establish a highly efficient preparation method for CVS-N2c-ΔG viruses. Here we describe a new preparation protocol involving three cell lines, as shown in Figure 1A: 1) the B7GG cell line (Osakada et al., 2011), based on BHK-21 cells stably expressing the T7 RNA polymerase, nuclear-localized EGFP, and the SAD-B19 glycoprotein (B19G), which was used for the rapid rescue and amplification of CVS-N2c-ΔG viruses; 2) the BHK-N2cG cell line (Additional Figure 1A), based on BHK-21 cells stably expressing the CVS-N2c glycoprotein (N2cG), along with nuclear-localized EGFP, used for the amplification of N2cG-coated CVS-N2c-ΔG viruses; and 3) the BHK-EnvARVG cell line (Additional Figure 1B), based on BHK-21 cells stably expressing the EnvA and B19G chimeric glycoprotein (EnvARVG) (Wickersham et al., 2007b) and nuclear-localized EGFP, used for the amplification of EnvARVG-coated CVS-N2c-ΔG viruses. We found that the CVS-N2c-ΔG virus was quickly rescued (Figure 1B).
To evaluate the production performance of these cell lines, we compared the production efficiency of CVS-N2c-ΔG and SAD-B19-ΔG viruses in these cells by supernatant titer detection. In B7GG cells, no significant difference was observed in the supernatant titer between CVS-N2c-ΔG and SAD-B19-ΔG viruses (SAD-B19-ΔG: 1.04 ± 0.26 × 106 IU/mL; CVS-N2c-ΔG: 0.88 ± 0.30 × 106 IU/mL; P = 0.6978) (Figure 1C). The supernatant titer of CVS-N2c-ΔG was lower in BHK-N2cG cells than that produced in the B7GG cell line (B7GG: 0.88 ± 0.30 × 106 IU/mL; BHK-N2cG: 1.04 ± 0.26 × 105 IU/mL; P = 0.0338) (Figure 1D). The SAD-B19-ΔG virus was also amplified in BHK-N2cG cells, and the supernatant titer was also significantly lower than that produced in the B7GG cell line (B7GG: 1.04 ± 0.26 × 106 IU/mL; BHK-N2cG: 1.24 ± 0.25 × 105 IU/mL; P = 0.0077) (Figure 1E). However, there was no significant difference in supernatant titer between CVS-N2c-ΔG and SAD-B19-ΔG produced in the same BHK-N2cG cell line (SAD-B19-ΔG: 1.24 ± 0.25 × 105 IU/mL; CVS-N2c-ΔG: 1.04 ± 0.26 × 105 IU/mL; P = 0.5917; Figure 1F).
Neuro2A-EnvARVG was inefficient in producing the CVS-N2c-ΔG virus (Reardon et al., 2016), which may be because EnvARVG itself is not suitable for CVS-N2C-ΔG virus packaging and may also be because of the low viability of Neuro2A cells. To verify whether the BHK-EnvARVG cell line can efficiently produce high-titer EnvARVG-coated CVS-N2C-ΔG, we used high-titer CVS-N2C-ΔG pseudotyped with B19G (produced by the B7GG cell line) to infect BHK-EnvARVG cells. As shown in Figure 1G, the supernatant titer was measured and compared with EnvARVG-pseudotyped SAD-B19-ΔG. No significant difference was detected in supernatant titer between CVS-N2c-ΔG and SAD-B19-ΔG produced in the same BHK-EnvARVG cell line (SAD-B19-ΔG: 7.46 ± 1.15 × 105 IU/mL; CVS-N2c-ΔG: 4.88 ± 0.97 × 105 IU/mL; P = 0.1242; Figure 1G), indicating that the BHK-EnvARVG cell line can be used to efficiently prepare a high-titer EnvARVG-pseudotyped CVS-N2C-ΔG virus.
Figure 2|Efficient retrograde labeling with the N2cG-coated CVS-N2c-ΔG virus.
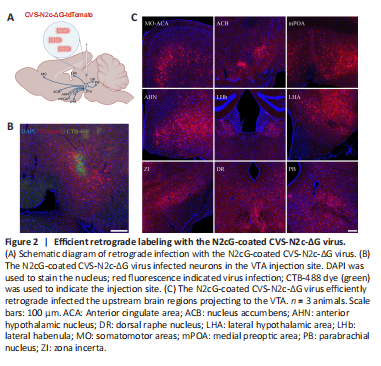
The retrograde labeling of viral tracers is used to analyze the upstream neural networks projected to specific brain regions. N2cG-coated SAD-B19-ΔG performs efficient retrograde labeling of the upstream network of specific brain regions along the axon terminal (Zhu et al., 2020), and CVS-N2c-ΔG has a lower cytotoxicity compared with SAD-B19-ΔG (Reardon et al., 2016). Therefore, if N2cG can endow the CVS-N2c-ΔG virus with efficient retrograde labeling, it will be more conducive to structural labeling and functional manipulation. To evaluate whether the N2cG-coated CVS-N2c-ΔG virus achieves high-efficiency retrograde labeling of projection neurons, N2cG-coated CVS-N2c-ΔG virus and CTB-488 were mixed and injected into the VTA of C57BL/6 adult mice (Figure 2A), and local infection and the brain regions projecting to the VTA were imaged. We found that the N2cG-coated CVS-N2c-ΔG virus labeled the neurons in situ (Figure 2B) and achieved retrograde labeling of the upstream brain area of the VTA (Montardy et al., 2019), including the somatomotor areas, anterior cingulate area, medial preoptic area, anterior hypothalamic nucleus, lateral habenula, lateral hypothalamic area, zona incerta, dorsal raphe nucleus, and parabrachial nucleus (Figure 2C). These results demonstrate that N2cG-coated CVS-N2c-ΔG virus allows efficient retrograde access to projection neurons.
Figure 3|Application of CVS-N2c-ΔG virus for efficient transduction in the VTA/SNc-to-DLS pathway.
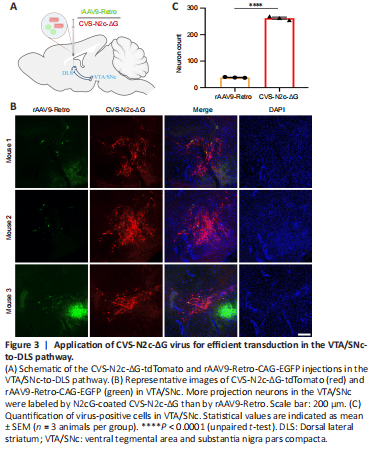
To demonstrate that the N2cG-coated CVS-N2c-ΔG virus allows efficient retrograde access to projection neurons that are difficult to label with other tools, we compared its efficiency with another retrograde viral tracer, rAAV9-Retro, which retrogradely infects projection neurons with an efficiency comparable to that of rAAV2-Retro (Tervo et al., 2016; Lin et al., 2020b). rAAV2-Retro and rAAV9-Retro exhibit robust retrograde functionality in certain neural circuits, but they have brain region selectivity and weak labeling efficiency in projection neurons from the VTA and substantia nigra pars compacta (VTA/SNc) to the dorsal lateral striatum (DLS) (Tervo et al., 2016; Li et al., 2018; Lin et al., 2020b). N2cG-coated CVS-N2c-ΔG-tdTomato and rAAV9-Retro-CAG-EGFP were mixed and injected into the CPu (DLS) of C57BL/6 adult mice (Figure 3A), and local infection and the VTA/SNc region projecting to the DLS were imaged. rAAV9-Retro showed a small amount of EGFP expression in the VTA/SNc. In contrast, N2cG-coated CVS-N2c-ΔG robustly drove tdTomato expression in projection neurons in the VTA/SNc (Figure 3B), demonstrating its efficient retrograde transport in the VTA/SNc to the DLS pathway. Importantly, we observed a statistically significant difference in the total number of positive neurons in the VTA/SNc between rAAV9-Retro and CVS-N2c-ΔG (38.33 ± 0.88 for rAAV9-Retro, 262.30 ± 4.06 for CVS-N2c-ΔG; P < 0.0001) (Figure 3C). These results indicate that the N2cG-coated CVS-N2c-ΔG allows efficient retrograde access to projection neurons unaddressed by rAAV9-Retro, and the efficiency is approximately six times higher than that of rAAV9-Retro.
Figure 4|Comparison of retrograde trans-monosynaptic efficiency following trans-complementation with oG.
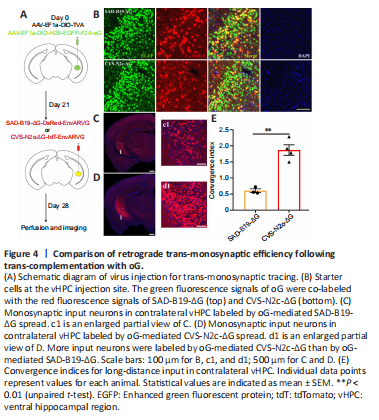
Rabies virus from different strains combined with different glycoproteins may have different trans-synaptic efficiency or another infection tropism. The oG derived from the Pasteur strain of the rabies virus greatly improved the trans-monosynaptic tracing efficiency of SAD-B19-ΔG (Kim et al., 2016). However, whether it can enhance CVS-N2c-ΔG trans-monosynaptic spread efficiency has not been examined. Therefore, we compared different retrograde trans-monosynaptic systems, as shown in Additional Figure 2, which mainly include rabies virus systems with a deletion of glycoproteins and AAV helper virus systems that compensate the TVA for specific infections and glycoproteins for trans-monosynaptic tracing. We first evaluated the “leaking” of EnvARVG-pseudotyped rabies viruses. EnvARVG-pseudotyped rabies viruses were each injected into the vHPC of C57BL/6 mice, and 1 week later, the brain slices were imaged. No red fluorescent signals were observed at the injection site, indicating that the EnvARVG-pseudotyped rabies viruses were rigorous (Additional Figure 3). To verify whether oG can enhance CVS-N2c-ΔG trans-monosynaptic spread efficiency, we used two helper viruses (AAVs) introduced in trans: one to complement the oG and the other to express TVA. The viruses were mixed and injected into the vHPC of Thy1-Cre transgenic mice (Campsall et al., 2002). After 3 weeks, CVS-N2c-ΔG and SAD-B19-ΔG were each injected at the same site. Seven days later, the brain slices were processed and imaged using a slide scanner (Figure 4A). We found that a number of nuclear GFP and dsRed neurons were co-labeled (starter cells) within the vHPC (Figure 4B), and rabies viruses efficiently trans-monosynaptically transduced the contralateral vHPC (Figure 4C and D). The trans-monosynaptic tracing efficiency of rabies virus was evaluated through the convergence index, which was calculated as the number of dsRed+ input neurons divided by the number of GFP+ dsRed+ starter neurons (Kim et al., 2016). The results showed that the trans-monosynaptic efficiency of oG-mediated CVS-N2c-ΔG was approximately three times higher than that of oG-mediated SAD-B19-ΔG (CVS-N2c-ΔG/oG: 1.87 ± 0.16; SAD-B19-ΔG/oG: 0.60 ± 0.06; P = 0.0014) (Figure 4E).
Figure 5|Neuronal activity monitoring in vivo with the CVS-N2c-ΔG virus.
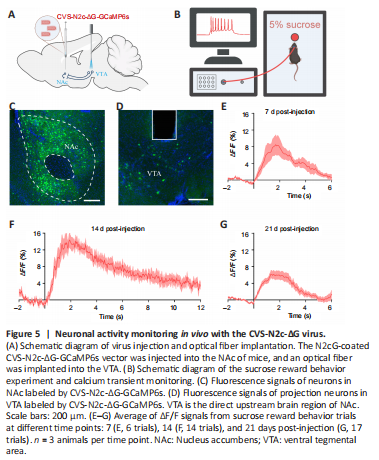
Although CVS-N2c-ΔG exhibits reduced toxicity to neurons compared with the SAD-B19-ΔG vaccine strain and can be used for the long-term neuronal activity monitoring of labeled circuits in vivo, it still has some neurotoxicity and the time for monitoring neural activity is limited to 17 days (Reardon et al., 2016). We thus evaluated these functional characteristics of the viruses produced by the new method. To evaluate whether projection neurons transduced with the N2cG-coated CVS-N2c-ΔG vector retained the properties for monitoring neural activity, and to determine the time window during which function detection is maintained, we established a CVS-N2c-ΔG-GCaMP6s viral vector and conducted in vivo response monitoring of the calcium transients of reward circuits at different time points. The projection neurons from the VTA to the NAc are involved in “reward circuits” (Mohebi et al., 2019). We thus performed fiber photometry in this projection pathway (Figure 5A) and used 5% sugar water as a reward. We injected the N2cG-coated CVS-N2c-ΔG-GCaMP6s vector into the NAc of mice and detected the changes in calcium signals in the VTA when mice were rewarded with sugar water (Figure 5A and B). We found that the projection pathway was labeled by GCaMP6s (Figure 5C and D) and activated when mice consumed sugar water at 7, 14, and 21 DPI (Figure 5E–G). These results indicate that N2cG-coated CVS-N2c-ΔG can be used to monitor the neural activity of projection neurons, and this can be maintained for at least 21 days.
Figure 6|Cre- and Flp-dependent recombination with the CVS-N2c-ΔG virus.
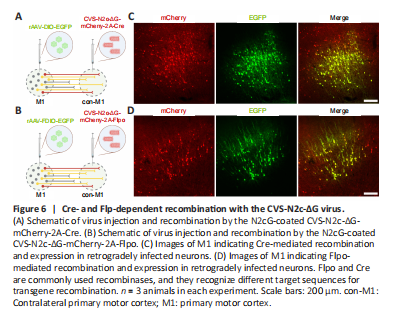
Recombinant enzyme-mediated gene expression or manipulation plays an important role in the study of neural circuit structure and function (Chatterjee et al., 2018). To verify whether the CVS-N2c-ΔG virus produced using the new method can effectively express a recombinant enzyme for the recombination of Cre- or Flpo-induced genes, for Cre-mediated recombination, Cre-conditional rAAV expressing EGFP (rAAV-DIO-EGFP) was injected into M1 of C57BL/6 adult mice, followed by an injection of CVS-N2c-ΔG-mCherry-2A-Cre at the contralateral M1 (Figure 6A). For Flpo-mediated recombination, Flpo-conditional rAAV expressing EGFP (rAAV-FDIO-EGFP) was injected into the M1 of C57BL/6 adult mice, followed by an injection of CVS-N2c-ΔG-mCherry-2A-Flpo at the contralateral M1 (Figure 6B). We found that both CVS-N2c-ΔG-mCherry-2A-Cre and CVS-N2c-ΔG-mCherry-2A-Flpo could transport retrogradely and drive EGFP expression of AAV, and the green fluorescence signals were co-labeled with the red fluorescence signals (Figure 6C and D). These results indicate that the CVS-N2c-ΔG virus produced using the new method can express sufficient recombinases for efficient transgene recombination, consistent with previous reports (Reardon et al., 2016).