神经退行性病
-
Figure 1|Increased expression of HTR3A in brains from Tg AD mice and AD patients.

To evaluate whether HTR3A is involved in AD pathology, we first examined HTR3A expression in Tg AD mice. Western blot analysis displayed that HTR3A levels were significantly increased in both the cortex and hippocampus in 3-month-old Tg AD mice (before the appearance of Aβ plaques (Krauthausen et al., 2015; Liu et al., 2020)) and 10-month-old Tg AD mice compared with age-matched WT mice (Figure 1A–C).
Immunofluorescence staining was used to examine the morphological features of HTR3A-positive cells. In 7-month-old WT mice (when Aβ plaques appeared in AD mice), HTR3A immunoreactivity was faint and punctate with a scattered pattern throughout the II–V layer of the cerebral cortex, stratum radium, stratum lacunosum-moleculare of the hippocampus, and molecular layer of the dentate gyrus; intact HTR3A-positive interneurons were not observed (Figure 1D). In age-matched Tg AD mice, HTR3A staining was remarkably intense and intact morphology of HTR3A-positive interneurons was observed, with thickened and shortened processes in hippocampus and cortex; some HTR3A interneurons were clustered (Figure 1D). Immunohistochemical staining also showed that HTR3A-positive interneurons were clustered with thickened and shortened processes in 10-month-old Tg AD mice while HTR3A immunoreactivity was weak in age-matched WT mice (Figure 1E).
To determine whether HTR3A interneurons are associated with Aβ plaques, we performed double thioflavin S/HTR3A staining and observed many clustered HTR3A interneurons around Aβ plaques. Notably, colocalization of HTR3A with thioflavin S was observed (Figure 1G), indicating that HTR3A interneurons may generate Aβ peptides. Additionally, double-immunostaining of APP/HTR3A and BACE1/HTR3A showed that some of the clustered APP- or BACE1-positive neurites near Aβ plaques colocalized with HTR3A interneurons (Figure 1H and I), indicating that some APP-positive or BACE1-positive neurites are derived from HTR3A interneurons. These results indicate that HTR3A-positive interneurons may contribute, at least in part, to Aβ generation in Tg AD mice.
Figure 2|Chronic tropisetron treatment diminishes Aβ plaque levels in Tg AD mice.
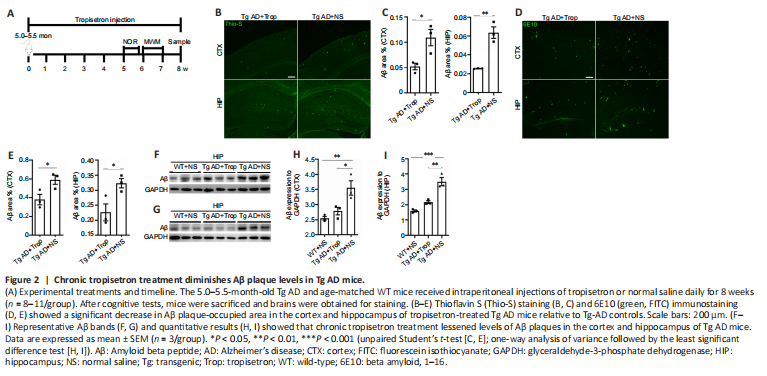
To investigate the mechanisms underlying the effects of chronic tropisetron treatment (Figure 2A), we measured the levels of Aβ plaques in Tg AD mice. Using thioflavin S staining and 6E10 immunostaining, we observed a significant decrease in Aβ plaque-occupied areas in the cortex and hippocampus of tropisetron-treated Tg AD mice relative to Tg-AD controls (Figure 2B–E). Immunoblot analysis also showed a significant decrease in Aβ levels in cortex and hippocampus of tropisetron-treated Tg AD mice compared with Tg-AD controls (Figure 2F–I). These findings suggest that chronic tropisetron treatment attenuated Aβ plaque levels in Tg AD mice.
Figure 3|Chronic tropisetron treatment mitigates glial activation in Tg AD mice.

Given that glial activation and ensuing neuroinflammation are highly associated with levels of Aβ plaques in AD pathogenesis, we investigated glial activation in Tg AD mice following chronic tropisetron treatment. Western blot analysis showed that chronic tropisetron treatment significantly inhibited IBA-1 expression in the hippocampus and cortex of Tg AD mice (Figure 3A–C). In addition, immunostaining analysis exhibited that chronic tropisetron treatment significantly decreased the amounts of microglia labeled by IBA-1 or CD68 in the cortex and hippocampus of Tg AD mice (Figure 3D–G). Furthermore, immunoblot analysis displayed that GFAP levels were significantly decreased in the hippocampus, but no significant decrease was observed in the cortex of Tg AD mice given tropisetron relative to the NS-treated Tg-AD group (Figure 3H–J). This was also confirmed by immunostaining analysis, showing a significant decrease in the amounts of GFAP-positive astrocytes in the hippocampus of tropisetron-treated Tg AD mice (Figure 3K and L). These results suggest that chronic tropisetron treatment relieves glial activation in Tg AD mice.
Figure 4|Chronic tropisetron treatment diminishes neuroinflammation in Tg AD mice.
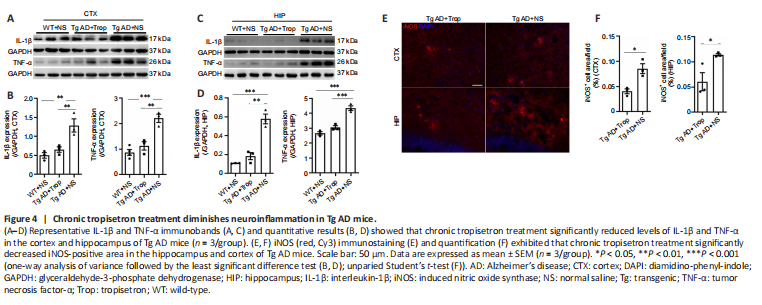
Glial activation is closely correlated with cytokine production (Liu et al., 2020). Thus, pro-inflammatory cytokines including IL-1β, TNF-α and iNOS were assessed. Immunoblot analysis displayed that levels of IL-1β and TNF-α in the hippocampus and cortex were significantly increased in Tg-AD mice compared with WT controls, consistent with a previous report (Liu et al., 2020). Notably, chronic tropisetron treatment significantly lowered the levels of IL-1β and TNF-α in Tg AD mice (Figure 4A–D). Moreover, iNOS immunostaining displayed that chronic tropisetron treatment significantly decreased the iNOS-positive area in the hippocampus and cortex of Tg AD mice (Figure 4E and F). These results show that chronic tropisetron treatment reduces neuroinflammation in Tg AD mice.
Figure 6|Chronic tropisetron treatment downregulates HTR3A expression in the hippocampus of Tg AD mice.

Receptors may be adjusted upwards or downwards after long-term administration of receptor blockers or agonists. Thus, we measured protein levels of HTR3A, which is required for functional HTR3 and determines HTR3 function. Western blot analysis showed that HTR3A levels of hippocampus and cortex in Tg AD mice were significantly increased compared with WT controls (Figure 6A–C), consistent with our results in Figure 1A. Notably, chronic tropisetron treatment significantly reduced the elevation of HTR3A expression in hippocampus but had no significant effect in the cortex of Tg AD mice (Figure 6A–C). Additionally, HTR3A immunostaining showed that the amounts of HTR3A-positive interneurons in tropisetron-treated Tg AD mice were significantly decreased in the hippocampus but no statistical difference was observed in the cortex compared with Tg-AD control (Figure 6D and E). These findings indicates that chronic tropisetron treatment significantly down-regulates HTR3A expression in the hippocampus of Tg AD mice.
Figure 7|Chronic tropisetron treatment partly prevents increased basal intracellular calcium (iCa2+) levels and decreases the number of iCa2+/HTR3A interneurons in Tg AD mice.
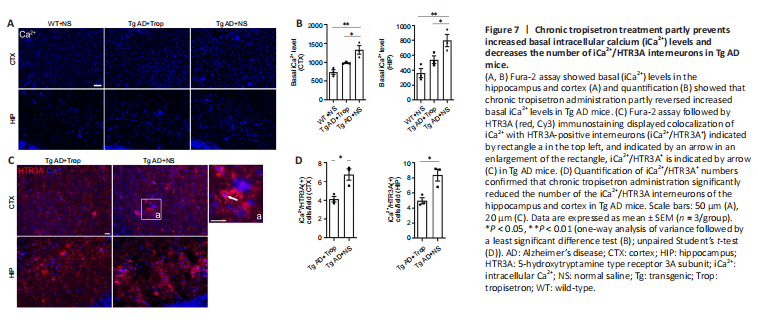
HTR3 is an ionotropic receptor with permeability to Ca2+ (Maricq et al., 1991; McMahon and Kauer, 1997; Turner et al., 2004). Thus, we assessed whether chronic tropisetron treatment affects basal levels of iCa2+ in HTR3A-positive interneurons in Tg AD mice. Basal iCa2+ levels were measured by Fura-2 assay as previously described (Doolen et al., 2012; Waldeck-Weiermair et al., 2015). Tg AD mice showed a significant increase in basal iCa2+ levels in the hippocampus and cortex compared with WT controls, and chronic tropisetron administration partly reversed the increased basal iCa2+ levels in Tg AD mice (Figure 7A and B). We next examined basal iCa2+ level in HTR3A-positive interneurons (iCa2+/HTR3A+) in tropisetron-treated AD mice and AD control using Fura-2 assay with HTR3A immunostaining. Notably, we observed colocalization of iCa2+ with some HTR3A-positive interneurons (iCa2+/HTR3A+) (Figure 7C), suggesting increased basal iCa2+ levels in HTR3A-positive interneurons. Quantitative analysis demonstrated that chronic tropisetron administration significantly lowered the number of the iCa2+/HTR3A+ interneurons in the hippocampus and cortex compared with that of Tg AD mice (Figure 7D). These results indicate that chronic tropisetron administration significantly reversed the increased basal iCa2+ levels and number of iCa2+/HTR3A+ interneurons in Tg AD mice.
Figure 8|Chronic tropisetron treatment inhibits calcineurin (CaN)/nuclear factor of activated T cells (NFAT) signaling in Tg AD mice.
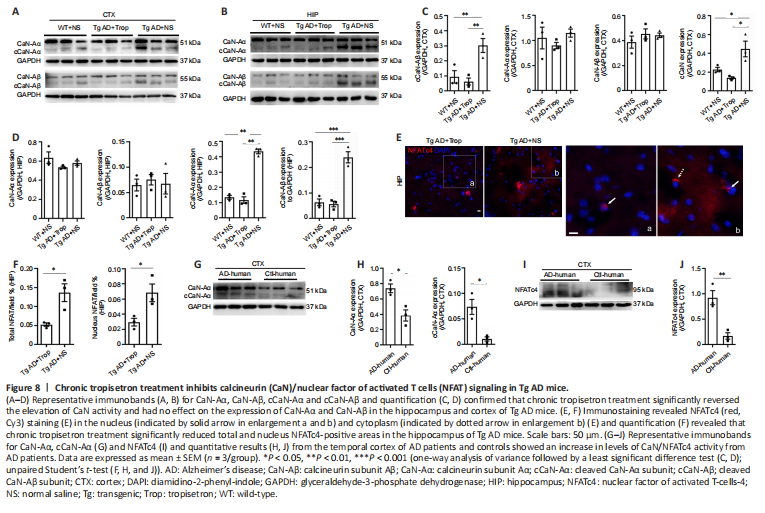
Given that tropisetron affected basal iCa2+ levels in Tg AD mice, we further examined whether iCa2+ downstream signaling was impacted. We examined the expression and activity of Calcineurin (CaN), a serine/threonine protein phosphatase exclusively regulated by iCa2+ and calmodulin (Rusnak and Mertz, 2000), in Tg AD mice after chronic tropisetron treatment. Western blot assay showed that the expressions of subunit Aα and Aβ of CaN in the hippocampus and cortex were similar in all groups of mice (Figure 8A–D). However, activated CaN, as indicated by cleaved truncated A and B subunit (cCaN-Aα and cCaN-Aβ), was significantly elevated in the hippocampus and cortex of Tg AD controls compared with WT controls (Figure 8A–D), confirming an elevated CaN activity in Tg AD mice, as previously reported (Norris et al., 2005). Notably, the elevated CaN activity was reversed by chronic tropisetron administration in Tg AD mice (Figure 8A–D). PKCα kinase, which is activated by iCa2+ and diacylglycerol (Mérida et al., 2019), and the calcium buffering protein calbindin were examined by western blot assay. No statistical difference was observed in p-PKCα and calbindin levels among the three groups (Additional Figure 3A–D).
NFATc (isoforms, i.e., NFATc 1–4), nuclear factor of activated T cells, are well-known CaN substrates (Abdul et al., 2009; Hopp et al., 2018). When dephosphorylated by CaN, NFATc leaves the cytosol and translocates into nucleus to regulate targeted gene expression (Abdul et al., 2009). Previous studies showed an increase in activated NFATc4 in brains from Tg AD mice and AD patients (Abdul et al., 2009; Hudry et al., 2012; Li et al., 2017; Hopp et al., 2018). Therefore, we examined NFATc4 using immunostaining analysis. As previously described (Hudry et al., 2012), fluorescence intensity of NFATc4 in nucleus was determined by overlap with the nuclear staining DAPI. NFATc4 positive staining was observed in both the nucleus and cytoplasm (Figure 8E). Quantitative analysis revealed that chronic tropisetron treatment significantly decreased both the total NFATc4-positive area and nuclear NFATc4-positive area in the hippocampus of Tg AD mice (Figure 8F). Furthermore, levels of CaN-Aα, active cCaN-Aα and NFATc4 were significantly increased in the temporal cortex of AD patients compared with controls, consistent with previous reports (Abdul et al., 2009; Hopp et al., 2018). The NF?κB transcriptional factor is implicated in neuroinflammation and subsequent cognitive impairment and is activated by phosphorylated IκB-α (Li et al., 2017). The levels of IκB-α and phosphorylated IκB-α in the hippocampus and cortex were not changed among the three mouse groups (Figure 8, and Additional Figure 3E–H). Together, these results suggest that chronic tropisetron treatment reversed the increase of CaN/NFAT signaling but had no significant effect on NF?κB signaling in Tg AD mice.