神经损伤与修复
-
Figure 1|Morphine induces HMGB1 release.
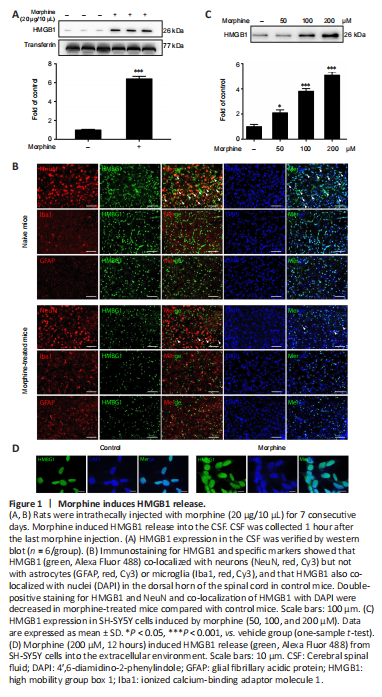
Rats were intrathecally injected with morphine (10 μg/10 μL) once daily for 7 consecutive days to establish the morphine tolerance model, after which CSF was collected to investigate whether morphine induced HMGB1 efflux into the extracellular environment. The western blot results showed that morphine caused marked release of HMGB1 into CSF (Figure 1A). To further investigate the cellular mechanism underlying morphine-induced HMGB1 release, we analyzed HMGB1 distribution by confocal microscopy. In the control mice, HMGB1 was mainly localized in neurons (NeuN-positive cells), not in astrocytes (GFAP-positive cells) or microglia (Iba-positive cells). In addition, HMGB1 localized to cell nuclei, reflecting HMGB1’s role as a non-histone nuclear protein. However, treatment with morphine resulted in a decrease in double-positive staining for HMGB1 and NeuN in the spinal cord of mice. Furthermore, the subcellular location of HMGB1 changed, strongly implying that morphine induced the release of HMGB1 from the nucleus into the extracellular CSF (Figure 1B). Next, the human neuroblastoma cell line SH-SY5Y, which expresses both mu- and delta-opioid receptors (at an approximate ratio of 4.5:1) (Yu et al., 1990) was utilized to provide in vitro confirmation of morphine-induced HMGB1 release. SH-SY5Y cells were incubated with different concentrations (50, 100, 200 μM) of morphine for 12 hours, and then the supernatants were analyzed by western blot. 3-(4,5)-Dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide assay confirmed that morphine did not affect cell proliferation at the different concentrations used (50, 100, and 200 μM) (Additional Figure 3). Western blotting showed that treatment with morphine induced HMGB1 excretion into the extracellular environment in a concentration-dependent manner (Figure 1C). Moreover, the immunofluorescence results showed that morphine caused HMGB1 to migrate from the nucleus to the cytoplasm (Figure 1D).
Figure 2|Glycyrrhizin attenuates chronic morphine tolerance.
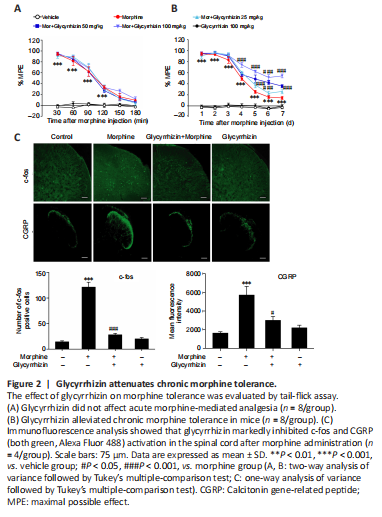
Given our observations indicating that morphine induced HMGB1 release, next we examined whether the released HMGB1 was important for morphine tolerance. As a selective HMGB1 inhibitor (Li et al., 2019), glycyrrhizin was utilized to evaluate the effects of the released HMGB1 on morphine tolerance. Glycyrrhizin did not enhance the acute analgesic effect of morphine (Figure 2A), and the behavioral test results revealed that glycyrrhizin suppressed chronic morphine tolerance in a dose-dependent manner (Figure 2B). On day 7, the MPE at 30 minutes decreased to 14.5% in chronic morphine-treated mice, whereas mice that received both glycyrrhizin (25, 50, and 100 mg/kg)
and morphine displayed MPEs of 26.3%, 35.9%, and 54.7%, respectively (Figure 2B). Moreover, high levels of c-fos (a marker of nociceptive neuron activation (Gao and Ji, 2009)) and CGRP (an indicator of pain (Benemei et al., 2009)) expression were observed in the dorsal horn of the spinal cord in mice with morphine tolerance, and this effect was inhibited by treatment with 50 or 100 mg/kg glycyrrhizin (Figure 2C).
Figure 3|Extracellular HMGB1 triggers an inflammatory response that is dependent on TLR4 expression in microglia.
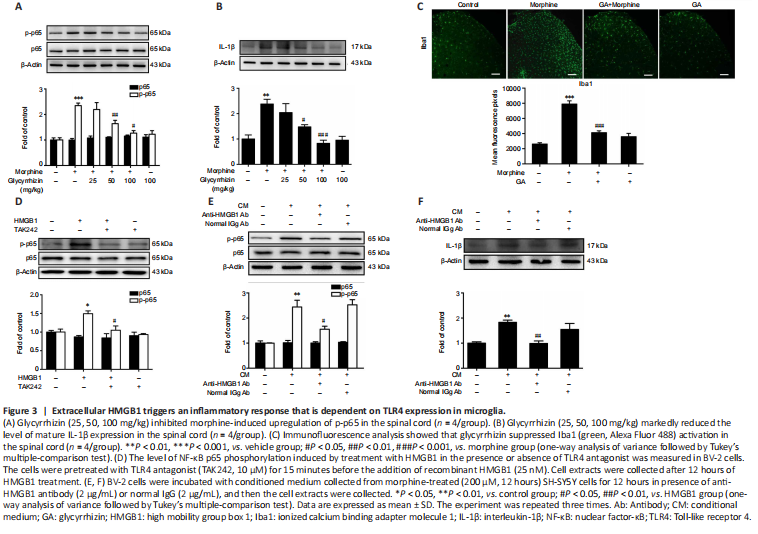
Next, we explored the role of the released HMGB1 in neuroinflammation. Microglia, which account for 10–15% of all cells in the central nervous system, are the resident immune cells in the central nervous system. When exposed to a variety of damaging stimuli, such as ischemia/hypoxia, trauma, and infection, microglia are rapidly activated and release a large number of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6 (Walter et al., 2007; Fernandez-Lizarbe et al., 2013; Deora et al., 2020). It has been reported that microglial TLR4 activated by DAMPs potentiates neuroinflammation through inflammasome-induced IL-1β expression. Cai et al. (2016) reported that morphine induces NLRP3 inflammasome activation and IL-1β maturation. We found that repeated morphine administration led to phosphorylation of NF-κB p65 and upregulation of the proinflammatory cytokine IL-1β in the spinal cord (Figure 3A and B). Glycyrrhizin attenuated these effects (Figure 3A and B). Moreover, immunofluorescence analysis showed that repeated morphine treatment led to an increase in Iba-1 expression, and that glycyrrhizin inhibited microgligal activation (Figure 3C). In order to confirm the involvement of microglia in morphine-induced NF-κB p65 phosphorylation and IL-1β upregulation in the spinal cord, we pretreated mice with PLX3397 to deplete microglia. Mice were treated with PLX3397 (40 mg/kg) for a total of 10 days starting 3 days before the first injection of morphine. The results showed that PLX3397 significantly depleted microglia in mice (Additional Figure 4A) and suppressed chronic morphine tolerance (Additional Figure 4B). Furthermore, western blot analysis showed that PLX3397 decreased NF-κB p65 phosphorylation and IL-1β expression in the spinal cord (Additional Figure 4C). These findings demonstrate that microglial activation is necessary for inducing morphine tolerance.
点击此处查看全文
We next verified the effect of extracellular HMGB1 on the inflammatory response using the immortalized murine microglial cell line BV-2 (Blasi et al., 1990; Wang et al., 2012). We found that treatment with recombinant HMGB1 (25 nM) significantly upregulated NF-κB p65 phosphorylation in BV-2 cells, whereas treatment with a TLR4 antagonist (TAK242, 10 μM) inhibited this effect (Figure 3D). These findings show that microglial activation triggered by HMGB1-TLR4 signaling is necessary for morphine tolerance. Finally, we collected conditioned medium from SH-SY5Y cells treated with morphine (200 μM, 12 hours) and used it to activate BV-2 cells to confirm the role of HMGB1 in triggering an inflammatory response. Anti-HMGB1 neutralizing antibody (2 μg/μL) decreased the NF-κB p65 phosphorylation and upregulation of IL-1β expression induced by the conditioned medium (Figure 3E and F). Normal IgG (2 μg/μL) did not have any significant effect (Figure 3E and F).